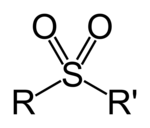
A sulfone is a chemical compound containing a sulfonyl functional group attached to two carbon atoms. The central sulfur atom is twice double bonded to oxygen and has two further hydrocarbon substituents. The general structural formula is R-S(=O)(=O)-R' where R and R' are the organic groups. The use of the alternative name sulphone is discouraged by IUPAC. Sulfides are often the starting materials for sulfones by organic oxidation through the intermediate formation of sulfoxides. For example dimethyl sulfide is oxidized to dimethyl sulfoxide and then to dimethyl sulfone.
In the Ramberg-Bäcklund Reaction and the Julia olefination sulfones are converted to alkenes.
A sulfone can also be any of various organic sulfur compounds having a sulfonyl group attached to two carbon atoms, especially such a compound formerly used as an antibiotic to treat leprosy, dermatitis herpetiformis, tuberculosis, or Pneumocystis Carinii Pneumonia (PCP).




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 192.111.xxx.xx
192.111.xxx.xx
 Server Time:
Server Time: