 |
|
|
Sulfonamide (medicine)
|
|
| Systematic (IUPAC) name | |
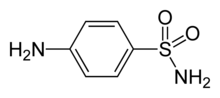
| sulfanilamide | |
| Identifiers | |
| CAS number | 63-74-1 |
| ATC code | J01EB06 D06BA05 |
| PubChem | 5333 |
| DrugBank | APRD00438 |
| Chemical data | |
| Formula | C6H8N2O2S |
| Mol. weight | 172.206 g/mol |
There are several sulphonamide-based groups of drugs. The original antibacterial sulfonamides (sometimes called simply sulfa drugs) are synthetic antimicrobial agents that contain the sulfonamide group. The sulfonylureas (main article: sulfonylureas) and thiazide diuretics (main article thiazide) are newer drug groups based on the antibacterial sulfonamides.
In bacteria, antibacterial sulfonamides act as competitive inhibitors of the enzyme dihydropteroate synthetase, DHPS. DHPS catalyses the coversion of PABA (para-aminobenzoate) to dihydropteroate, a key step in folate synthesis. Folate is necessary for the cell to synthesize nucleic acids (nucleic acids are essential building blocks of DNA and RNA), and in its absence cells will be unable to divide. Hence the sulfonamide antibacterials exhibit a bacteriostatic rather than bactericidal effect.
Folate is not synthesized in mammalian cells, but is instead a dietary requirement. This explains the selective toxicity to bacterial cells of these drugs.
Sulfa allergies are common, hence medications containing sulfonamides are prescribed carefully.
Contents |
History
Sulfonamide drugs (known widely as "sulfa drugs") were the first antibacterial antibiotics, and paved the way for the antibiotic revolution in medicine. The first sulfonamide was trade named Prontosil, which is a prodrug. Experiments with Prontosil began in 1932 in the laboratories of the Bayer Corporation, a component of the huge German chemical trust IF Farben. The dye-based drug was synthesized by Bayer chemist Josef Klarer and tested in animals under the direction of physician/researcher Gerhard Domagk. Domagk quickly won the 1939 Nobel Prize in Medicine and Physiology, an honor that Hitler forbade him to accept (Hitler was incensed at the awarding of a Nobel Peace Prize a few years earlier to an anti-Nazi activist). Domagk's Prize also caused some bad feeling between him and Klarer. The discovery was in fact a team effort in which Domagk played a central role, operating under the general direction of Heinrich Hoerlein, a Farben executive later tried (and acquited) at Nuremberg. The first official communication about the breakthrough discovery was not published until 1935, more than two years after the drug was patented by Klarer and his research partner Fritz Mietzsch. Prontosil was the first medicine ever discovered that could effectively treat a range of bacterial infections inside the body. It had a strong protective action against infections caused by streptococci, including blood infections, childbed fever, and erysipelas, and a lesser effect on infections caused by other cocci. Perplexingly, it had no effect at all in the test tube, exerting its antibacterial action only in live animals. Later it was discovered by a French research team at the Pasteur Institute that the drug was metabolized into two pieces inside the body, releasing from the inactive dye portion a smaller, colorless, active compound called sulfanilamide. The discovery helped establish the concept of "bioactivation" and dashed the German corporation's dreams of enormous profit; the active molecule sulfanilamide (or sulfa) had first been synthesized in 1906 and was widely used in the dye-making industry; its patent had since expired and the drug was available to anyone.
The result was a sulfa craze. For several years in the late 1930s hundreds of manufacturers produced tens of thousands of tons of myriad forms of sulfa. Unfortunately one of them, a preparation called Elixir Sulfanilamide, was made with a toxic solvent, diethylene glycol. The Elixir killed more than one hundred people, mostly children, in the US, and led to the passage of the 1938 Food, Drug, and Cosmetic Act. As the first and only only effective antibiotic available in the years before penicillin, sulfa drugs continued to thrive through the early years of World War II, saving the lives of Winston Churchill, Franklin Delano Roosevelt's son, and tens of thousands of patients. Sulfa had a central role in preventing wound infections during the war. American soldiers were issued a first aid kit containing sulfa powder and were told to sprinkle it on any open wound. During the years 1942 to 1943, Nazi doctors conducted sulfanilamide experiments on prisoners in concentration camps.
The sulfanilamide compound is more active in the protonated form, which in case of the acid works better in a basic environment. The solubility of the drug is very low and sometimes can crystalize in the kidneys, due to its first pKa of around 10. This is a very painful experience so patients are told to take the medication with copious amounts of water. Newer compounds have a pKa of around 5-6 so the problem is avoided.
Many thousands of molecules containing the sulfanilamide structure have been created since its discovery (by one account, over 5400 permutations by 1945), yielding improved formulations with greater effectiveness and less toxicity. Sulfa drugs are still widely used for conditions such as acne and urinary tract infections, and are receiving renewed interest for the treatment of infections caused by bacteria resistant to other antibiotics.
Sulpha is an alternate (UK English) spelling of the common name for Sulfonamide antibiotics.
Preparation
Sulfonamides are prepared by the reaction of a sulfonyl chloride with ammonia or an amine. Certain sulfonamides (sulfadiazine or sulfamethoxazole) are sometimes mixed with the drug trimethoprim, which acts against dihydrofolate reductase.
Side Effects
Sulfonamides have the potential to cause a variety of untoward reactions, including urinary tract disorders, haemopoietic disorders, porphyria and hypersensitivity reactions. When used in large dose, it may develop a strong allergic reaction.
See also
External links
- http://www.thomashager.net - author of "The Demon under the Microscope," a history of the discovery of the sulfa drugs
- http://www.lung.ca/tb/tbhistory/treatment/chemo.html - A History of the Fight Against Tuberculosis in Canada (Chemotherapy)
- http://www.nobel.se/medicine/laureates/1939/press.html - Lecture, Nobel Prize in Physiology and Medicine, 1939
- http://home.att.net/~steinert/wwii.htm - The History of WW II Medicine
- http://www.life.umd.edu/classroom/bsci424/Chemotherapy/AntibioticsHistory.htm - A history of antibiotics




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xxx
216.73.xxx.xxx
 Server Time:
Server Time: