| Sodium hypochlorite | |
|---|---|
 |
|
| General | |
| Other names | Sodium chlorate(I) |
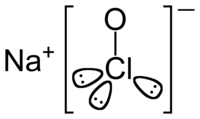
| Molecular formula | NaClO |
| Molar mass | 74.44 g/mol |
| Appearance | white solid |
| CAS number | [7681-52-9] |
| Properties | |
| Density and phase | 1.07-1.14 g/cm^3 liquid |
| Solubility in water | Fully miscible |
| Melting point | 18░C Pentahydrate |
| Boiling point | 101░C Decomposes |
| Hazards | |
| EU classification | Corrosive (C) Dangerous for the environment (N) |
| R-phrases | R31, R34, R50 |
| S-phrases | S1/2, S28, S45, S50, S61 |
| Related compounds | |
| Other anions |
Sodium chloride Sodium chlorite Sodium chlorate Sodium perchlorate |
| Other cations |
Lithium hypochlorite Calcium hypochlorite |
| Related compounds | Hypochlorous acid |
|
Except where noted otherwise, data are given
for materials in their standard state (at 25 ░C, 100 kPa) |
|
Sodium hypochlorite is a chemical compound with the formula NaClO. A solution of sodium hypochlorite is frequently used as a disinfectant and as a bleaching agent; indeed, often it is simply called "bleach", though other chemicals are sometimes given that name as well.
Contents |
Production
Sodium hypochlorite may be prepared by absorbing chlorine gas in cold sodium hydroxide solution:
- 2NaOH + Cl2 → NaCl + NaClO + H2O
Sodium hydroxide and chlorine are commercially produced by the chloralkali process, and there is no need to isolate them to prepare sodium hypochlorite. Hence NaClO is prepared industrially by the electrolysis of sodium chloride solution without any separation between the anode and the cathode. The solution must be kept below 40 ░C (by cooling coils) to prevent the formation of sodium chlorate.
The commercial solutions always contain significant amounts of sodium chloride (common salt) as the main byproduct, as seen in the equation above.
Packaging and sale
Household bleach sold for use in laundering clothes is a 3-6% solution of sodium hypochlorite at the time of manufacture. Strength varies from one formulation to another and gradually decreases with long storage.
A 12% solution is widely used in waterworks for the chlorination of water. High-test hypochlorite (HTH) is sold for chlorination of swimming pools and contains approximately 30% sodium hypochlorite. The crystalline salt is also sold for the same use; this salt usually contains less than 50% of sodium hypochlorite. However, the level of "active chlorine" may be much higher.
Uses
In household bleach form, sodium hypochlorite is used for removal of stains from laundry. It is particularly effective on cotton fiber, which stains easily but bleaches well. 50 to 250 ml per load is usually recommended for a standard-size washer. Hot water increases the activity of the bleach, owing to the thermal decomposition of hypochlorite which ultimately generates environmentally-undesirable chlorate.
A weak solution of 1% household bleach in warm water is used to sanitize smooth surfaces prior to brewing of beer or wine. Surfaces must be rinsed to avoid imparting flavors to the brew; these chlorinated byproducts of sanitizing surfaces are also harmful.
A 1 in 5 dilution of household bleach with water (1 part bleach to 4 parts water) is effective against many bacteria and some viruses, and is often the disinfectant of choice in cleaning surfaces in hospitals (Primarily in the United States). The solution is corrosive, and needs to be thoroughly removed afterwards, so the bleach disinfection is sometimes followed by an ethanol disinfection.
For shock chlorination of wells or water systems, a 2% solution of household bleach is used. For larger systems, HTH is more practical because lower rates can be used. The alkalinity of the sodium hypochlorite solution also causes the precipitation of minerals such as calcium carbonate, so that the shock chlorination is often accompanied by a clogging effect. The precipitate also preserves bacteria, making this practice somewhat less effective.
Sodium hypochlorite has been used for the disinfection of drinking water, at a concentration equivalent to about 1 liter of household bleach per 4000 liters of water is used. The exact amount required depends on the water chemistry, temperature, contact time, and presence or absence of sediment. In large-scale applications, residual chlorine is measured to titrate the proper dosing rate. For emergency disinfection, the US EPA recommends the use of 2 drops of 5%ac household bleach per quart of water. If the treated water doesn't smell of bleach, 2 more drops are to be added.
The use of chlorine-based disinfectants in domestic water, although widespread, has led to some controversy due to the formation of small quantities of harmful byproducts such as chloroform.
It is also used in dentistry, during root canal treatment, disinfecting the canal and dissolving any remaining pulp tissue. Historically, Henry Drysdale Dakin's solution (0.5%) had been used. Nowadays, 2.5-5.25% solutions are being used.
An alkaline solution (pH 11.0) of sodium hypochlorite is used to treat dilute (< 1 g/L) cyanide wastewater, e.g. rinsewater from an electroplating shop. In batch treatment operations, sodium hypochlorite has been used to treat more concentrated cyanide wastes, such as silver cyanide plating solutions. A well-mixed solution is fully treated when an excess of chlorine is detected.
Mechanism of action
Like all hypochlorites, sodium hypochlorite is a salt of hypochlorous acid, HClO. Sodium hypochlorite solution is a light yellow green transparent liquid. In water, it partially splits into the sodium cation Na+ and the hypochlorite anion ClO−, while a substantial portion hydrolyses into sodium hydroxide and hypochlorous acid. The oxidizing power of the latter and of the hypochlorite anion cause the bleaching effect. The hypochlorite anion's negative charge, however, prevents it from diffusing through the cell walls of bacteria and microbes, making it a poor disinfectant. However, the hypochlorous acid molecules that exist in equilibrium with the hypochlorite anion, due to their neutral charge and small size, easily diffuse through the cell walls of bacteria. This changes the oxidation-reduction potential (ORP) of the cell, and inactivates the enzyme triosephosphate dehydrogenase. Triosephosphate dehydrogenase (or glyceraldehyde 3-phosphate dehydrogenase/GAPDH) is essential for the digestion of glucose, but is particularly sensitive to oxidizing agents. Its inactivation effectively destroys the micro-organism's ability to function.
Cautions
Sodium hypochlorite is a strong oxidizer. Products of the oxidation reactions are corrosive. Solutions burn skin and cause eye damage, particularly when used in concentrated forms. However, as recognized by the NFPA, only solutions containing more than 40% sodium hypochlorite by weight are considered hazardous oxidizers. Solutions less than 40% are classified as a moderate oxidizing hazard (NFPA 430, 2000).
Household bleach and pool chlorinator solutions are typically stabilized by a significant concentration of lye (caustic soda, NaOH) as part of the manufacturing reaction. Skin contact will produce caustic irritation or burns due to defatting and saponification of skin oils and destruction of tissue. The slippery feel of bleach on skin is due to this process.
Sodium thiosulfate (hypo) is an effective chlorine neutralizer. Rinsing with a 5mg/L solution, followed by washing with soap and water, quickly removes chlorine odor from the hands.
Chlorination of drinking water can oxidize organic contaminants, producing trihalomethanes (also called haloforms), which are carcinogenic. The extent of the hazard thus created is a subject of disagreement.
Mixing bleach with some household cleaners can be hazardous. For example, mixing an acid cleaner with sodium hypochlorite bleach generates chlorine gas. Mixing with ammonia solutions produces chloramines. Both chlorine gas and chloramine gas are toxic. Bleach can react violently with hydrogen peroxide and produce oxygen gas:
- H2O2 + NaClO → NaCl + H2O + O2
It is estimated that there are about 3300 accidents needing hospital treatment caused by sodium hypochlorite solutions each year in British homes (RoSPA, 2002).
Bibliography
- Jones, F.-L. (1972). "Chlorine poisoning from mixing household cleaners". J. Am. Med. Assoc. 222: 1312.
- Institut National de Recherche et de SÚcuritÚ. (2004). "Eaux et extraits de Javel. Hypochlorite de sodium en solution". Fiche toxicologique n░ 157, Paris.
External links
- International Chemical Safety Card 0482 (solutions <10% active Cl)
- International Chemical Safety Card 1119 (solutions >10% active Cl)
- European Chemicals Bureau
- Institut National de Recherche et de SÚcuritÚ (in French)
- Home and Leisure Accident Statistics 2002 (UK RoSPA)
- Emergency Disinfection of Drinking Water (US EPA)
- Chlorinated Drinking Water (IARC Monograph)
- NTP Study Report TR-392: Chlorinated & Chloraminated Water (US NIH)




 216.73.216.2
216.73.216.2 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.x
216.73.xxx.x
 Server Time:
Server Time: