| Naphthalene | |
|---|---|
 |
|
| General | |
| Chemical name | Naphthalene |
| Other names | Tar Camphor, White Tar, Moth Flakes |
| Chemical formula | C10H8 |
| SMILES | C1(C=CC=C2)=C2C=CC=C1 |
| Molar mass | 128.17052 g/mol |
| Appearance | White solid crystals/flakes, strong odor of coal tar |
| CAS number | 91-20-3 |
| Properties | |
| Density | 1.14 g/cm³ |
| Solubility in water | Insoluble in water |
| Melting point | 80.5 °C |
| Boiling point | 218 °C |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | Flammable, sensitizer, possible carcinogen. Dust can form explosive mixtures with air |
| NFPA 704 |

2
2
0
|
| Flash point | 79 - 87 °C |
| Autoignition temperature | 525 °C |
| R/S statement | R: 20, 21, 22, 36, 37, 38, 43, 45 S: 16, 26, 36, 37, 39, 45 |
| RTECS number | QJ0525000 |
|
Except where noted otherwise, data are given
for materials in their standard state (at 25°C, 100 kPa) |
|
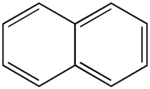
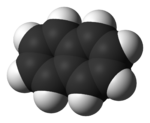
Naphthalene (not to be confused with naphtha) (also known as naphthalin, naphthaline, tar camphor, white tar, albocarbon, or naphthene), is a crystalline, aromatic, white, solid hydrocarbon, best known as the primary ingredient of mothballs. Naphthalene is volatile, forming a flammable vapor. Its molecules consist of two fused benzene rings. It is manufactured from coal tar, and converted to phthalic anhydride for the manufacture of plastics, dyes and solvents. It is also used as an antiseptic and insecticide, especially in mothballs. p-Dichlorobenzene is now often used instead of naphthalene as a mothball substitute. Naphthalene easily sublimates at room temperature.
Contents |
History
In 1819-1820, at least two chemists reported a white solid with a pungent odor derived from the distillation of coal tar. In 1821, John Kidd described many of this substance's properties and the means of its production, and proposed the name naphthaline, as it had been derived from a kind of naphtha (a broad term encompassing any volatile, flammable liquid hydrocarbon mixture, including coal tar).
Naphthaline's empirical formula, C5H4, was determined by Michael Faraday in 1826. The structure of two fused benzene rings was proposed by Emil Erlenmeyer in 1866, and confirmed by Carl Graebe three years later.
Structure and reactivity
A naphthalene molecule is composed of two fused benzene rings. (In organic chemistry, rings are fused if they share two or more atoms). Accordingly, naphthalene is classified as a benzenoid polycyclic aromatic hydrocarbon (PAH). Naphthalene has three resonance structures, which are shown in the drawing below. Naphthalene has two sets of equivalent hydrogens. The alpha positions are positions 1, 4, 5, and 8 on the drawing below. The beta positions are positions 2, 3, 6, and 7.
Unlike benzene, the carbon-carbon bonds in naphthalene are not of the same length. The bonds C1–C2, C3–C4, C5–C6 and C7–C8 are about 1.36 Å (136 pm) in length, whereas all the other carbon-carbon bonds are about 1.42 Å (142 pm) in length. This has been verified by x-ray diffraction and can be expected from the resonance structures, where the bonds C1–C2, C3–C4, C5–C6 and C7–C8 are double in two of the three structures, whereas all the others are double in only one.

Like benzene,naphthalene can undergo electrophilic aromatic substitution. For many electrophilic aromatic substitution reactions, naphthalene is more reactive than benzene, and reacts under milder conditions than does benzene. For example, while both benzene and naphthalene react with chlorine in the presenece of a ferric chloride or aluminium chloride catalyst, naphthalene and chlorine can react to form 1-chloronaphthalene even without a catalyst. Similarly, while both benzene and naphthalene can be alkylated using Friedel-Crafts reactions, naphthalene can also be alkylated by reaction with alkenes or alcohols, with sulfuric or phosphoric acid as the catalyst.
Mono-substitution of naphthalene has two possible isomeric products, corresponding to substitution at an alpha or beta position, respectively. Usually, the major product has the electrophile in the alpha position. The selectivity for alpha over beta substitution can be rationalized in terms of the resonance structures of the intermediate: for the alpha substitution intermediate, seven resonance structures can be drawn, of which four preserve an aromatic ring. For beta substitution, the intermediate has only six resonance structures, and only two of these are aromatic. Sulfonation, however, gives a mixture of the "alpha" product 1-naphthalenesulfonic acid and the "beta" product 2-naphthalenesulfonic acid, with the ratio dependent on reaction conditions.
Naphthalene can be hydrogenated under high pressure or with a suitable catalyst to give 1,2,3,4-tetrahydronaphthalene, a solvent sold under the trade name Tetralin. Further hydrogenation yields decahydronaphthalene or Decalin (C10H18, also known as bicyclo[4.4.0]decane). Oxidation of naphthalene with chromate or permanganate, or catalytic oxidation with O2 and a vanadium catalyst, gives phthalic acid.
Production
Most naphthalene is derived from coal tar. From the 1960s until the 1990s, significant amounts of naphthalene were also produced from heavy petroleum fractions during petroleum refining, but today petroleum-derived naphthalene represents only a minor component of naphthalene production.
Naphthalene is the most abundant single component of coal tar. While the composition of coal tar varies with the coal from which it is produced, typical coal tar is about 10% naphthalene by weight. In industrial practice, distillation of coal tar yields an oil containing about 50% naphthalene, along with a variety of other aromatic compounds. This oil, after being washed with aqueous sodium hydroxide to remove acidic components, chiefly various phenols, and with sulfuric acid to remove basic components, is fractionally distilled to isolate naphthalene. The crude naphthalene resulting from this process is about 95% naphthalene by weight. The chief impurity is the sulfur-containing aromatic compound thionaphthene. Petroleum-derived naphthalene is usually purer than that derived from coal tar. Where purer naphthalene is required, crude naphthalene can be further purified by recrystallizing it from any of a variety of solvents.
Incidence in nature
Trace amounts of naphthalene are produced by magnolias and specific types of deer. Naphthalene has also been found in the Formosan subterranean termite, possibly as a repellant against "ants, poisonous fungi and nematode worms." [1]
Uses
Naphthalene's most familiar use is as a household fumigant, such as in mothballs. In a sealed container containing naphthalene pellets, naphthalene vapors build up to levels toxic to both the adult and larval forms of many moths that are destructive to textiles. Other fumigant uses of naphthalene include use in soil as a fumigant pesticide, and in attic spaces to repel animals.
In the past, naphthalene was administered orally to kill parasitic worms in livestock.
Larger volumes of naphthalene are used as a chemical intermediate to produce other chemicals. The single largest use of naphthalene is the industrial production of phthalic anhydride, although more phthalic anhydride is made from o-xylene than from naphthalene. Other naphthalene-derived chemicals include alkyl naphthalene sulfonate surfactants, and the insecticide carbaryl. Naphthalenes substituted with combinations of strongly electron-donating functional groups, such as alcohols and amines, and strongly electron-withdrawing groups, especially sulfonic acids, are intermediates in the preparation of many synthetic dyes. The hydrogenated naphthalenes tetrahydronaphthalene (Tetralin) and decahydronaphthalene (Decalin) are used as low-volatility solvents.
Health effects
In humans, exposure to large amounts of naphthalene may damage or destroy red blood cells. This could cause the body to have too few red blood cells until it replaces the destroyed cells. Humans, particularly children, have developed this condition after ingesting mothballs or deodorant blocks containing naphthalene. Some of the symptoms of this condition are fatigue, lack of appetite, restlessness, and pale skin. Exposure to large amounts of naphthalene may also cause nausea, vomiting, diarrhea, blood in the urine, and jaundice (yellow coloration of the skin).
When the U.S. National Toxicology Program exposed male and female rats and mice to naphthalene vapors on weekdays for two years (1), male and female rats exhibited: evidence of carcinogenic activity, based on increased incidences of adenoma and neuroblastoma of the nose, female mice exhibited some evidence of carcinogenic activity, based on increased incidences of alveolar and bronchiolar adenomas of the lung, and male mice exhibited no evidence of carcinogenic activity.
The International Agency for Research on cancer (IARC) (2) classifies naphthalene as possibly carcinogenic to humans [Group 2B]. It also points out that acute exposure causes cataracts in humans, rats, rabbits, and mice and, that haemolytic anaemia, described above, can occur in children and infants after oral or inhalation exposure or after maternal exposure during pregnancy.
Over 400 million people have an inherited condition called glucose-6-phosphate dehydrogenase deficiency. For these people, exposure to naphthalene is harmful and may cause hemolytic anemia, which causes their erythrocytes to break down.
References
- NTP Technical Reports 410 and 500. NTP Technical Reports 410 and 500, available from NTP: Long-Term Abstracts & Reports. Retrieved on March 6, 2005.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Monographs on the Evaluation of Carcinogenic Risks to Humans, Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene, Vol. 82 (2002) (p. 367). Retrieved on March 9, 2005.
- John Kidd (1821). "Obersvations on Naphthaline, a peculiar substance resembling a concrete essential oil, which is apparently produced during the decomposition of coal tar, by exposure to a red heat". Philosophical Transactions 111: 209-221.
External links
- Naphthalene (PIM 363) - mostly on toxicity of naphthalene




 216.73.216.103
216.73.216.103 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xxx
216.73.xxx.xxx
 Server Time:
Server Time: