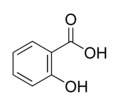
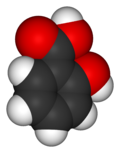
| Salicylic acid | |
|---|---|
| Chemical name | 2-Hydroxybenzoic acid |
| Chemical formula | C7H6O3 |
| Molecular mass | 138.12 g/mol |
| Melting point | 159 °C |
| Boiling point | 211 °C (2666 Pa) |
| Density | 1.44 g/cm3 (at 20 °C) |
| pKa | 2.97 |
| CAS number | [69-72-7] |
| SMILES | c1(O)ccccc1C(O)=O |
 |
|
| Disclaimer and references | |
Salicylic acid is the chemical compound with the formula C6H4(OH)CO2H, where the OH group is adjacent to the carboxylic acid group. This colorless crystalline organic acid is widely used in organic synthesis and functions as a plant hormone. It is probably best known as a compound that is chemically similar but not identical to the active component of aspirin. The name derives from the latin word for the willow tree (Salix), from whose bark it can be obtained.
Contents |
Medicinal and cosmetic uses
Also known as beta hydroxy acid (compare to AHA), salicylic acid is the key additive in many skin-care products for the treatment of acne, psoriasis, callouses, corns, keratosis pilaris and warts. It treats acne by causing skin cells to slough off more readily, preventing pores from clogging up. This effect on skin cells also makes salicylic acid an active ingredient in several shampoos meant to treat dandruff. Use of straight salicylic solution may cause hyperpigmentation on unpretreated skin for those with darker skin types (Fitzpatrick phototypes IV, V, VI), as well as with the lack of use of a broad spectrum sunblock.[1][2]
The medicinal properties of salicylate (mainly for fever relief) have been known since ancient times. The substance occurs in the bark of willow trees; the name salicylic acid is derived from salix, the Latin name for the willow tree. [3]

Aspirin (acetylsalicylic acid or ASA) can be prepared by the esterification of the phenolic hydroxyl group of salicylic acid.
Subsalicylate in combination with bismuth form the popular stomach relief aid known commonly as Pepto-Bismol. When combined, the two key ingredients help control diarrhea, nausea, heartburn, and gas. It is also a very mild anti-biotic.
Toxicological effects of 100% salicylic acid, however, are mostly harmful. It is harmful by ingestion, inhalation, and through skin absorption. It acts as an irritant, and chronic effects have shown 100% salicylic acid to cause DNA damage, and also cause allergic reactions after repeated exposure. This is why most acne treatment medications use a percent range of 2-5 in solution.
Other uses
- Salicylic acid is toxic if ingested in large quantities, but in small quantities is used as a food preservative and antiseptic in toothpaste. For some people with salicylate sensitivity even these small doses can be harmful.
See also
Footnotes
- ^ Grimes P.E. (1999). "The Safety and Efficacy of Salicylic Acid Chemical Peels in Darker Racial-ethnic Groups". Dermatologic Surgery 25: 18-22.
- ^ Roberts W. E. (2004). "Chemical peeling in ethnic/dark skin". Dermatologic Therapy 17 (2): 196. DOI:10.1111/j.1396-0296.2004.04020.x.
- ^ Philip A. Mackowiak (2000). "Brief History of Antipyretic Therapy". Clinical Infectious Diseases, 31: 154–156. DOI:10.1086/317510.
References
- http://www.chemexper.com/. Retrieved on July 18, 2005.
- http://www.inchem.org/. Retrieved on July 18, 2005.
- http://www.jtbaker.com/. Retrieved on July 18, 2005.
- Salicylic acid. Retrieved on July 18, 2005.




 216.73.216.2
216.73.216.2 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.x
216.73.xxx.x
 Server Time:
Server Time: