 |
|
 |
|
|
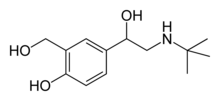
Salbutamol
|
|
| Systematic (IUPAC) name | |
|
2-(hydroxymethyl)-4-[1-hydroxy-
2-(tert-butylamino)ethyl]phenol |
|
| Identifiers | |
| CAS number | 18559-94-9 |
| ATC code | R03AC02 R03CC02 |
| PubChem | 2083 |
| DrugBank | APRD00553 |
| Chemical data | |
| Formula | C13H21NO3 |
| Mol. weight | 239.311 |
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Half life | 1.6 hours |
| Excretion | Urinary |
| Therapeutic considerations | |
| Pregnancy cat. | A(AU) C(US) |
| Legal status | S3(AU) ?(CA) POM(UK) ℞-only(US) |
| Routes | Oral, inhalational, IV |
Salbutamol (INN) or albuterol (USAN) is a short-acting β2-adrenergic receptor agonist used for the relief of bronchospasm in conditions such as asthma and COPD.
Salbutamol sulfate is usually given by the inhaled route for direct effect on bronchial smooth muscle. This is usually achieved through a metered dose inhaler (MDI), nebuliser or other proprietary delivery devices (e.g. Rotahaler or Autohaler). Salbutamol can also be given orally or intravenously. However, some asthmatics may not respond to these medications as they will not have the required DNA base sequence in a specific gene.
Salbutamol became available in the United Kingdom in 1969 and in the United States in 1980 under the trade name Ventolin.
Contents |
Clinical use
Salbutamol is specifically indicated in the following conditions:
- acute asthma
- symptom relief during maintenance therapy of asthma and other conditions with reversible airways obstruction (including COPD)
- protection against exercise-induced asthma
- hyperkalemia, especially in patients with renal failure
- can be aerosolized for patients with Cystic Fibrosis, along with ipratropium bromide and pulmozyme.
As a β2-agonist, salbutamol also finds use in obstetrics. Intravenous salbutamol can be used as a tocolytic to relax the uterine smooth muscle to delay premature labour. Whilst preferred over agents such as atosiban and ritodrine, its role has largely been replaced by the calcium-channel blocker nifedipine which is more effective, better tolerated and orally administered. (Rossi, 2004)
Mode of action
As with other β2-adrenergic receptor agonists, salbutamol binds to β2-adrenergic receptors with a higher affinity than β1-receptors. In the airway, activation of β2-receptors results in relaxation of bronchial smooth muscle resulting in a widening of the airway (bronchodilation). Inhaled salbutamol sulfate has a rapid onset of action, providing relief within 5-15 minutes of administration.
In tocolysis, the activation of β2-receptors results in relaxation of uterine smooth muscle, thus delaying labour.
Adverse effects
Whilst salbutamol is well-tolerated, particularly when compared with previous therapies such as theophylline, like all medications there exists the potential for adverse drug reactions to occur - especially when in high doses, or when taken orally or intravenously.
Common adverse effects include: tremor, palpitations and headache. (Rossi, 2004)
Infrequent adverse effects include: tachycardia, muscle cramps, agitation, hypokalemia, hyperactivity in children, and insomnia. (Rossi, 2004)
Other brand names
Salbutamol is sold under the brand names Airomir, Asthalin, Asmol, Buventol, Proventil, Salamol, Sultanol, Ventolin and Volmax.
References
- Rossi S (Ed.) (2004). Australian Medicines Handbook 2004 (AMH). Adelaide: Australian Medicines Handbook. ISBN 0-9578521-4-2.
External links
- Volmax Drug Information (drugs.com)




 216.73.216.81
216.73.216.81 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xx
216.73.xxx.xx
 Server Time:
Server Time: