 |
|
|
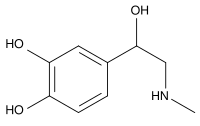
Epinephrine
|
|
| Systematic (IUPAC) name | |
|
4-(1-hydroxy- 2-(methylamino)ethyl)benzene-1,2-diol |
|
| Identifiers | |
| CAS number | 51-43-4 |
| ATC code | A01AD01 B02BC09 C01CA24 R01AA14 R03AA01 S01EA01 |
| PubChem | 838 |
| DrugBank | APRD00450 |
| Chemical data | |
| Formula | C9H13NO3 |
| Mol. weight | 183.204 g/mol |
| Pharmacokinetic data | |
| Bioavailability | Nil (oral) |
| Metabolism | adrenergic synapse (MAO and COMT) |
| Half life | 2 minutes |
| Excretion | n/a |
| Therapeutic considerations | |
| Pregnancy cat. | A(AU) C(US) |
| Legal status | S4(AU) POM(UK) ℞-only(US) |
| Routes | IV, IM, endotracheal |
Epinephrine (INN) (IPA: [ˌɛpɪˈnɛfrən]) or adrenaline (BAN) (IPA: [əˈdrɛnələn]), sometimes spelled "epinephrin" or "adrenalin" respectively, is a hormone. Epinephrine is a catecholamine, a sympathomimetic monoamine derived from the amino acids phenylalanine and tyrosine. The Latin roots ad-+renes and the Greek roots epi-+nephros both literally mean "on/to the kidney" (referring to the adrenal gland, which secretes epinephrine). Epinephrine is sometimes shortened to epi in medical jargon. Epinephrine is now also used in EpiPens. EpiPens are long narrow auto-injectors that administer epinephrine.
In May 1886, William Bates reported the discovery of a substance produced by the adrenal gland in the New York Medical Journal. Epinephrine was isolated and identified in 1895 by Napoleon Cybulski, a Polish physiologist. The discovery was repeated in 1897 by John Jacob Abel. Jokichi Takamine discovered the same hormone in 1900, without knowing about the previous discovery. It was first artificially synthesized in 1904 by Friedrich Stolz.
Contents |
Actions in the body
Epinephrine plays a central role in the short-term stress reaction—the physiological response to threatening, exciting or environmental stressor conditions such as high noise levels or bright light (see Fight-or-flight response). It is secreted by the adrenal medulla. When released into the bloodstream, epinephrine binds to multiple receptors and has numerous effects throughout the body. It increases heart rate and stroke volume, dilates the pupils, and constricts arterioles in the skin and gut while dilating arterioles in leg muscles. It elevates the blood sugar level by increasing depolymerization of glycogen to glucose in the liver, and at the same time begins the breakdown of lipids in adipocytes. Epinephrine has a suppressive effect on the immune system.
Epinephrine is used as a drug to promote peripheral vascular resistance via alpha-stimulated vasoconstriction in cardiac arrest and other cardiac dysrhythmias resulting in diminished or absent cardiac output, such that blood is shunted to the body's core. This beneficial action comes with a significant negative consequence—increased cardiac irritability—which may lead to additional complications immediately following an otherwise successful resuscitation. Alternatives to this treatment include vasopressin, a powerful antidiuretic which also increases peripheral vascular resistance leading to blood shunting via vasoconstriction, but without the attendant increase to myocardial irritability.
Because of its suppressive effect on the immune system, epinephrine is used to treat anaphylaxis and sepsis. Allergy patients undergoing immunotherapy may receive an epinephrine rinse before the allergen extract is administered, thus reducing the immune response to the administered allergen. It is also used as a bronchodilator for asthma if specific beta2-adrenergic receptor agonists are unavailable or ineffective. Adverse reactions to epinephrine include palpitations, tachycardia, anxiety, headache, tremor, hypertension, and acute pulmonary edema.
A pheochromocytoma is a tumor of the adrenal gland (or, rarely, the ganglia of the sympathetic nervous system), which secretes excessive amounts of catecholamines, usually epinephrine.
Pharmacology
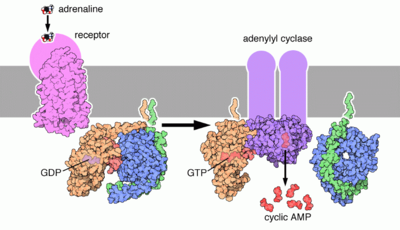
Epinephrine's actions are mediated through adrenergic receptors (sometimes referred to as adrenoceptors).
It binds to α1 receptors of liver cells, which activate inositol-phospholipid signaling pathway, signaling the phosphorylation of insulin, leading to reduced ability of insulin to bind to its receptors.
Epinephrine also activates β-adrenergic receptors of the liver and muscle cells, thereby activating the adenylate cyclase signaling pathway, which will in turn increase glycogenolysis. β2 receptors are found primarily in skeletal muscle blood vessels where they do indeed trigger vasodilation. However Alpha receptors are found in most smooth muscles and splanchnic vessels, and epinephrine triggers vasoconstriction in those vessels. Thus, depending on the patient, administration of epinephrine may raise or lower blood pressure, depending whether or not the net increase or decrease in peripheral resistance can balance the positive inotropic and chronotropic effects of epinephrine on the heart.
Terminology
Although widely referred to as "adrenaline" outside of the US, and the lay public worldwide, the USAN and INN for this chemical is "epinephrine" because "adrenaline" bore too much similarity to the Parke, Davis & Co trademark "adrenalin" (without the "e") which was registered in the US.
The BAN and EP term for this chemical is "adrenaline", and is indeed now one of the few differences between the INN and BAN systems of names.
Amongst US health professionals the term epinephrine is generally used over adrenaline. However, it should be noted that when referring to pharmaceuticals that mimic the actions of epinephrine/adrenaline their receptor sites are universally referred to as "adrenergics".
Epinephrine also known as adrenaline can be found in nature in the R form; however, racemic mixtures of the molecule can be used if its chirality is destroyed.
References
- Aronson JK (2000). "Where name and image meet" - the argument for "adrenaline". British Medical Journal 320, 506-9.
External links
- Action of Epinephrine on a Liver Cell (Animation)




 216.73.216.81
216.73.216.81 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xx
216.73.xxx.xx
 Server Time:
Server Time: