| Nitrous oxide | ||
|---|---|---|
|
||
| General | ||
| Molecular formula | N2O | |
| Molar mass | 44.0128 g/mol | |
| Appearance | colourless gas | |
| CAS number | [10024-97-2] | |
| Properties | ||
| Density and phase | 1.2 kg m−3 (liquid) | |
| Melting point | −90.86 °C (182.29 K) | |
| Boiling point | −88.48 °C (184.67 K) | |
| Structure | ||
| Molecular shape | linear | |
| Dipole moment | 0.166 D | |
| Thermodynamic data | ||
|
Std enthalpy of formation ΔfH |
+82.05 kJ/mol | |
| Hazards | ||
| MSDS | External MSDS | |
| EU classification | Oxidising (O) | |
| NFPA 704 |
|
|
| R-phrases | R8 | |
| S-phrases | S38 | |
| Related compounds | ||
| Related nitrogen oxides |
Nitric oxide Nitrogen dioxide Dinitrogen trioxide Dinitrogen tetroxide Dinitrogen pentoxide Nitric acid Nitrous acid |
|
| Related compounds |
Nitric acid Nitrous acid |
|
|
Except where noted otherwise, data are given
for materials in their standard state (at 25 °C, 100 kPa) |
||
Nitrous oxide, also known as dinitrogen oxide or dinitrogen monoxide, is a chemical compound with chemical formula N2O. Under room conditions, it is a colourless non-flammable gas, with a pleasant, slightly-sweet odor. It is used in surgery and dentistry for its anaesthetic and analgesic effects, where it is commonly known as laughing gas due to the euphoric effects of inhaling it. It is also used as an oxidizer in internal combustion engines. In this use it is known as nitrous, or NOS after a well-known brand which has become a genericized trademark. Nitrous oxide is present in the atmosphere where it acts as a powerful greenhouse gas.
Contents |
Chemistry
The structure of the nitrous oxide molecule is a linear chain of a nitrogen atom bound to a second nitrogen atom, which in turn is bound to an oxygen atom. It can be considered a resonance hybrid of N≡N+−O− and −N=N+=O.
Nitrous oxide, N2O, should not be confused with the other oxides of nitrogen such as nitric oxide NO and nitrogen dioxide NO2.
Nitrous oxide is isoelectronic with carbon dioxide.
It can be prepared by heating ammonium nitrate in the laboratory and can be used to produce nitrites by mixing it with boiling alkali metals, and to oxidize organic compounds at high temperatures.
The CAS number of nitrous oxide is 10024-97-2 and its UN number is 1070.
History
The gas was discovered by Joseph Priestley in 1772, who called it phlogisticated nitrous air (see phlogiston). Humphry Davy in the 1790s tested the gas on himself and some of his friends, including the poets Samuel Taylor Coleridge and Robert Southey. They soon realised that nitrous oxide considerably dulled the sensation of pain, even if the inhaler were still semi-conscious. And so it came into use as an anaesthetic, particularly by dentists, who do not typically have access to the services of an anesthesiologist and who may benefit from a patient who can respond to verbal commands.
Manufacture
Nitrous oxide is most commonly made by fusing and "boiling" ammonium nitrate to form steam, nitrous oxide, nitrogen, ammonium nitrate 'fog' and small amounts of very toxic higher oxides of nitrogen; (NO2, NO, etc):
- NH4NO3 → N2O + 2H2O, ΔH = −36.8 kJ:
The addition of various phosphates favors formation of a purer gas. This reaction occurs at around 240 °C, a temperature where ammonium nitrate is a moderately sensitive explosive and a very powerful oxidizer (perhaps on the order of fuming nitric acid). At temperatures much above 240 °C the exothermic reaction may run away, perhaps up to the point of detonation. The mixture must be cooled to avoid such a disaster. In practice, the reaction involves a series of tedious adjustments to control the temperature to within a narrow range, which it will not naturally tend to stay in. Professionals have destroyed whole neighborhoods by losing control of such commercial processes. Examples include the Ohio Chemical debacle in Montreal, 1966 and the Air Products & Chemicals, Inc. disaster in Delaware City, Delaware, 1977.
The direct oxidation of ammonia may someday rival the ammonium nitrate pyrolysis synthesis of nitrous oxide mentioned above. This capital-intensive process, which originates in Japan, uses a manganese dioxide-bismuth oxide catalyst. (Suwa et al. 1961; Showa Denka Ltd.)
- 2NH3 + 2O2 → N2O + 3H2O:
Higher oxides of nitrogen are formed as impurities. Note that uncatalyzed ammonia oxidation (i.e. combustion or explosion) goes primarily to N2 and H2O. The Ostwald process oxidizes ammonia to nitric oxide (NO), using platinum; this is the beginning of the modern synthesis of nitric acid from ammonia (see above).
Nitrous oxide can be made by heating a solution of sulfamic acids and nitric acids. A lot of gas was made this way in Bulgaria (Brozadzhiew & Rettos, 1975).
- HNO3 + NH2SO3H → N2O + H2SO4 + H2O:
There is no explosive hazard in this reaction if the mixing rate is controlled. However, as usual, toxic higher oxides of nitrogen form.
Colorless solutions of hydroxylamine hydrochloride and sodium nitrite may also be used to produce N2O.
- (NH3OH+Cl-) + NaNO2 → N2O + NaCl + H2O:
If the nitrite is added to the hydroxylamine solution, the gas produced is pure enough for inhalation, and the only remaining byproduct is salt water. However, if the hydroxylamine solution is added to the nitrite solution (nitrite is in excess), then toxic higher oxides of nitrogen form are produced.
Uses
Inhalant effects — laughing gas

Nitrous oxide (N2O) is a dissociative that can cause analgesia, euphoria, dizziness, flanging of sound, and, in some cases, slight hallucinations and mild aphrodisiac effect.
Aerosol propellant
The gas is licensed for use as a food additive (also known as E942), specifically as an aerosol spray propellant. Its most common uses in this context are in aerosol whipped cream canisters and as an inert gas used to displace staleness-inducing oxygen when filling packages of potato chips and other similar snack foods.
The gas is extremely soluble in fatty compounds. In aerosol whipped cream, it is dissolved in the fatty cream until it leaves the can, when it becomes gaseous and thus creates foam. Used in this way, it produces whipped cream four times the volume of the liquid, whereas whipping air into cream only produces twice the volume. If air were used as a propellant, under increased pressure the oxygen would accelerate rancidification of the butterfat, while nitrous oxide inhibits such degradation.
Rocket motors
Nitrous oxide can be used as an oxidizer in a rocket engine. This has the advantages over other oxidizers that it is non-toxic and, due to its stability at room temperature, easy to store and relatively safe to carry on a flight.
Nitrous oxide has been the oxidizer of choice in several hybrid rocket designs (using solid fuel with a liquid or gaseous oxidizer). The combination of nitrous oxide with hydroxyl-terminated polybutadiene fuel has been used by SpaceShipOne and others. It is also notably used in amateur and high power rocketry with various plastics as the fuel. An episode of MythBusters featured a hybrid rocket built using a paraffin/powdered carbon mixture (and later salami) as its solid fuel and nitrous oxide as its oxidizer.
Nitrous oxide can also be used in a monopropellant rocket. In the presence of a heated catalyst, N2O will decompose exothermically into nitrogen and oxygen, at a temperature of approximately 1300° Celsius. In a vacuum thruster, this can provide a monopropellant specific impulse (Isp) of as much as 180s. While noticeably less than the Isp available from hydrazine thrusters (monopropellant or bipropellant with nitrogen tetroxide), the decreased toxicity makes nitrous oxide an option worth investigating.
Internal combustion engine
In racing, nitrous oxide (often just "nitrous" in this context) is sometimes injected into the intake manifold (or prior to the intake manifold; some systems directly inject it into the cylinder) to increase power: even though the gas itself is not flammable, it delivers more oxygen than atmospheric air by breaking down at elevated temperatures, thus allowing the engine to burn more fuel and air. Additionally, since nitrous oxide is stored as a liquid, the evaporation of liquid nitrous oxide in the intake manifold causes a large drop in intake charge temperature. This results in a denser charge, and can reduce detonation, as well as increase power available to the engine.
The same technique was used during by World War II Luftwaffe aircraft with the GM 1 system to boost the power output of aircraft engines. Originally meant to provide the Luftwaffe standard aircraft with superior high-altitude performance, technological considerations limited its use to extremely high altitudes. Accordingly, it was only used by specialized planes like high-altitude reconnaissance aircraft, high-speed bombers and high-altitude interceptors.
One of the major problems of using nitrous oxide in a reciprocating engine is that it can produce enough power to destroy the engine. Power increases of 100–300% are possible, and unless the mechanical structure of the engine is reinforced, the engine may not survive this kind of operation.
It is very important with nitrous oxide augmentation of internal combustion engines to maintain temperatures and fuel levels so as to prevent preignition, or detonation (sometimes referred to as knocking or pinging).
Safety
The major safety hazards of nitrous oxide come from the fact that it is a compressed liquified gas, and a dissociative anaesthetic.
While normally inert in storage and fairly safe to handle, nitrous oxide can decompose energetically and potentially detonate if initiated under the wrong circumstances. Liquid nitrous oxide acts as a good solvent for many organic compounds; liquid mixtures can form somewhat sensitive explosives. Contamination with fuels has been implicated in a handful of rocketry accidents, where small quantities of nitrous / fuel mixtures detonated, triggering the explosive decomposition of residual nitrous oxide in plumbing.
Nitrous oxide in an atmosphere
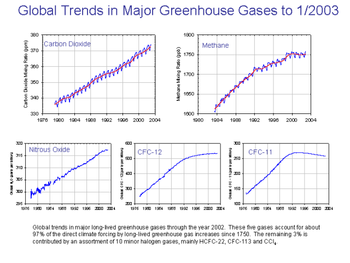
Unlike the other Nitrogen oxides, nitrous oxide is a major greenhouse gas; per unit of weight, nitrous oxide has 296 times the effect of carbon dioxide (CO2) for producing global warming [1]. Nitrous oxide is thus part of efforts to curb greenhouse gas emissions, such as the Kyoto Protocol. Behind carbon dioxide and methane, which has 23 times the greenhouse warming potential of carbon dioxide (over 100 years), nitrous oxide is the third most important gas that contributes to global warming. (The other nitrogen oxides contribute to global warming indirectly, by contributing to tropospheric ozone production during smog formation).
Nitrous oxide also attacks ozone in the stratosphere, aggravating the excess amount of UV striking the earth's surface in recent decades (various freons and related halogenated organics also consume ozone in the stratosphere). In the pre-industrial atmosphere nitrous oxide was (and remains) the main natural regulator of stratospheric ozone.
Nitrous oxide is naturally emitted by bacteria in soils and oceans. Agriculture is the main source of human-produced nitrous oxide: cultivating soil, the use of nitrogen fertilizers, and animal waste handling can all stimulate naturally occurring bacteria to produce more nitrous oxide. Industrial sources make up only about 20% of all anthropogenic sources, and include the production of nylon and nitric acid and the burning of fossil fuel in internal combustion engines.
Human activity is thought to account for somewhat less than 2 teragrams (this is multiplied by about 300 when calculated as a ratio to carbon dioxide) of nitrogen oxides per year, nature for over 15 teragrams [2]. The global anthropogenic nitrous oxide flux is about 1 petagram of carbon dioxide carbon-equivalents per year; this compares to 2 petagrams of methane carbon dioxide carbon-equivalents per year, and to an atmospheric loading rate of about 3.3 petagrams of carbon dioxide carbon-equivalents per year.
External links
- Paul Crutzen Interview Freeview video of Paul Crutzen Nobel Laureate for his work on decomposition of ozone talking to Harry Kroto Nobel Laureate by the Vega Science Trust.
- National Pollutant Inventory - Oxides of nitrogen fact sheet
- Nitrous Oxide Specs Extremely thorough Nitrous Oxide Facts




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 45.171.xx.xxx
45.171.xx.xxx
 Server Time:
Server Time:

