| Chloroform | |
|---|---|
  |
|
| General | |
| Other names | Trichloromethane Methane trichloride R-20 |
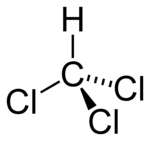
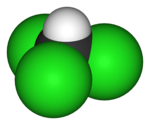
| Molecular formula | CHCl3 |
| Molar mass | 119.4 g/mol |
| Appearance | colorless liquid |
| SMILES | ClC(Cl)Cl |
| CAS number | [67-66-3] |
| EINECS number | 200-663-8 |
| Properties | |
| Density and phase | 1.48 g/cm³, liquid |
| Solubility in water | 0.8 g/100 ml at 20 °C |
| Melting point | −64 °C |
| Boiling point | 62 °C |
| Viscosity | 0.542 cP at 25 °C |
| Structure | |
| Molecular shape | Tetrahedral |
| Dipole moment | 1.08 D (gas) |
| Thermodynamic data | |
|
Standard enthalpy of formation ΔfH°liquid |
−134.3 kJ/mol |
|
Standard enthalpy of formation ΔfH°gas |
−103.2 kJ/mol |
|
Standard molar entropy S°gas |
295.6 J.K–1.mol–1 |
| Safety data | |
| EU classification |
Harmful Irritant Carc. Cat. 3 |
| R-phrases |
R22, R38, R40 R48/20/22 |
| S-phrases | S2, S36/37 |
| NFPA 704 |

0
2
0
|
| PEL-TWA (OSHA) | 50 ppm (240 mg/m3) |
| IDLH (NIOSH) | approx. 500 ppm |
| Flash point | non-flammable |
| RTECS number | FS9100000 |
| Supplementary data page | |
| Thermodynamic data | Phase behaviour Solid, liquid, gas |
| Related compounds | |
| Related Haloforms |
Fluoroform Bromoform Iodoform |
| Related Chloromethanes |
Chloromethane Dichloromethane Carbon tetrachloride |
|
Except where noted otherwise, data are given
for materials in their standard state (at 25 °C, 100 kPa) |
|
Chloroform, also known as trichloromethane and methyl trichloride, is a chemical compound with formula CHCl3. It does not support combustion in air, although it will burn when mixed with more flammable substances. It is a member of a subset of environmental pollutants known as trihalomethanes, a by-product of chlorination of drinking water and a long-standing health concern.
Contents |
History
Chloroform was discovered in July, 1831 by American physician Samuel Guthrie (1782-1848), and independently a few months later by French chemist Eugène Soubeiran (1797-1859) and Justus von Liebig (1803-1873) in Germany. Soubeiran produced chloroform through the action of chlorine bleach powder (calcium hypochlorite) upon acetone (propanone) or ethanol (an application of the generic process known as the haloform reaction). Chloroform was named and chemically characterised in 1834 by Jean-Baptiste Dumas (1800-1884). Its anaesthetic properties were noted early in 1847 by Marie-Jean-Pierre Flourens (1794-1867) and Robert James Fegle (1790-1842).
In 1847, the Edinburgh obstetrician James Young Simpson first used chloroform for general anesthesia during childbirth. The use of chloroform during surgery expanded rapidly thereafter in Europe. In the United States, chloroform began to replace ether as an anesthetic at the beginning of the 20th century; however, it was quickly abandoned in favor of ether upon discovery of its toxicity, especially its tendency to cause fatal cardiac arrhythmia analogous to what is now termed "sudden sniffer's death". Ether is still the preferred anesthetic in some developing nations due to its high therapeutic index and low price. Trichloroethylene, a halogenated aliphatic hydrocarbon related to chloroform, was proposed as a safer alternative, though it, too, was later found to be carcinogenic.
Production
Industrially, chloroform is produced by heating a mixture of chlorine and either chloromethane or methane to 400-500°C. At this temperature, a free radical halogenation occurs, converting the methane or chloromethane to progressively more chlorinated compounds.
- CH4 + Cl2 → CH3Cl + HCl
- CH3Cl + Cl2 → CH2Cl2 + HCl
- CH2Cl2 +Cl2 → CHCl3 + HCl
- CHCl3 + Cl2 → CCl4 + HCl
The output of this process is a mixture of the four chloromethanes, chloromethane, dichloromethane, chloroform (trichloromethane), and carbon tetrachloride, which are then separated by distillation.
The first industrial process was the reaction of acetone (or ethanol) with sodium hypochlorite or calcium hypochlorite. The chloroform can be removed from the resulting sodium acetate or calcium acetate (or sodium formate or calcium formate if ethanol is the starting material) by distillation. The reaction mechanism is called haloform reaction, and is still used for the production of bromoform and iodoform.
This reaction can also occur inadvertently when cleaning around the house. Sodium hypochlorite solution (bleach) and acetone (nail-varnish remover) produces chloroform, sodium hydroxide, sodium acetate, and sodium chloride. There have been reported cases of this method being used in the UK to synthesise chloroform in the home.
Deuterated chloroform is prepared by the reaction of sodium deuteroxide with chloral hydrate. Some of the aldehyde hydrogen is retained in the product, though, and samples of higher isotopic purity are obtained from trichloroacetophenone as starting material.
Uses
In the late 19th and early 20th centuries, chloroform was used as an inhaled anesthetic during surgery. However, safer, more flexible drugs have entirely replaced it in this role. The major use of chloroform today is in the production of the freon refrigerant R-22. However, as the Montreal Protocol takes effect, this use can be expected to decline as R-22 is replaced by refrigerants that are less liable to result in ozone depletion.
Smaller amounts of chloroform are used as a solvent in the pharmaceutical industry and for producing dyes and pesticides. It is used as a solvent for research in academic chemistry laboratories, also. As a solvent it can be used to bond pieces of acrylic glass (which is also known under the trade name 'Perspex'). Chloroform is one of the most effective known solvents for alkaloids in base form, and may be used to extract nitrogenous chemicals from plant material for pharmaceutical processing. It is commercially used to extract morphine from poppies, scopolamine from Datura plants, and so on.
Chloroform reacts with aqueous sodium hydroxide (preferably in the presence of a phase transfer catalyst) to produce dichlorocarbene. This is used to effect ortho-formylation of activated aromatic rings such as phenols, producing aryl aldehydes in a reaction known as the Reimer-Tiemann reaction. Alternatively the carbene may be trapped by an alkene to form a cyclopropane derivative.
Chloroform containing deuterium (heavy hydrogen), CDCl3, is the most common solvent used in NMR spectroscopy.
Safety
As might be expected from its use as an anesthetic, inhaling chloroform vapors depresses the central nervous system. Breathing about 900 parts of chloroform per million parts air (900 parts per million) for a short time can cause dizziness, fatigue, and headache. Chronic chloroform exposure may cause damage to the liver (where chloroform is metabolized to phosgene) and to the kidneys, and some people develop sores when the skin is immersed in chloroform. Approximately 10% of the population has an allergic reaction to chloroform that produces a fever of around 40°C (104°F) upon exposure.
Animal studies have shown that miscarriages occur in rats and mice that have breathed air containing 30 to 300 ppm chloroform during pregnancy and also in rats that have ingested chloroform during pregnancy. Offspring of rats and mice that breathed chloroform during pregnancy have a higher incidence of birth defects, and abnormal sperm have been found in male mice that have breathed air containing 400 ppm chloroform for a few days. The effect of chloroform on reproduction in humans is unknown.
Chloroform once appeared in toothpastes, cough syrups, ointments, and other pharmaceuticals, but it has been banned in consumer products in the United States since 1976.
The NTP's eleventh report on carcinogens implicates it as reasonably anticipated to be a human carcinogen, a designation equivalent to IARC class 2A. It has been most readily associated with hepatocellular carcinoma. Caution is mandated during its handling in order to minimize unnecessary exposure; safer alternatives, such as dichloromethane, have resulted in a substantial reduction of its use as a solvent.
During prolonged storage hazardous amounts of phosgene can accumulate in the presence of oxygen and ultraviolet light. To prevent accidents commercial material is stabilized with ethanol or amylene, but samples that have been recovered or dried no longer contain any stabilizer and caution must be taken with those. Suspicious bottles should be tested for phosgene. Filter paper strips, wetted with 5% diphenylamine, 5% dimethylaminobenzaldehyde, and then dried, turn yellow in phosgene vapor.
Due to its volatile nature, laboratory work with chloroform should be performed under a fume hood to avoid inhaling its fumes. Another problem with its volatility is that it can be difficult to pipette.
External links
- Chloroform: The molecular lifesaverAn article at Oxford University providing interesting facts about chloroform.
- Concise International Chemical Assessment Document 58
- History of chloroform anesthesia
- IARC Monograph on Chloroform
- International Chemical Safety Card 0027
- National Pollutant Inventory - Chloroform and trichloromethane
- NIOSH Pocket Guide to Chemical Hazards
- NIST Standard Reference Database
- Story on Chloroform from BBC's The Material World (28 July 2005)
- Sudden Sniffer's Death Syndrome article at Carolina Poison Center




 216.73.216.103
216.73.216.103 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xxx
216.73.xxx.xxx
 Server Time:
Server Time: