 |
|
|
Naproxen
|
|
| Systematic (IUPAC) name | |
|
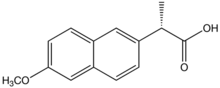
(+)-(S)-2-(6-methoxynaphthalen-2-yl) propanoic acid |
|
| Identifiers | |
| CAS number | 22204-53-1 |
| ATC code | G02CC02 M01AE02, M02AA12 |
| PubChem | 1302 |
| DrugBank | APRD01135 |
| Chemical data | |
| Formula | C14H14O3 |
| Mol. weight | 230.259 g/mol |
| Pharmacokinetic data | |
| Bioavailability | 95% (oral) |
| Protein binding | 99% |
| Metabolism | Hepatic (to 6-desmethylnaproxen) |
| Half life | 12–15 hours |
| Excretion | Renal |
| Therapeutic considerations | |
| Pregnancy cat. | C(AU) B(US) |
| Legal status | S2(AU)
POM(UK)
OTC(US) ℞-only(Ca) |
| Routes | Oral |
Naproxen (INN) (IPA: [nəˈprɒksən]) is a non-steroidal anti-inflammatory drug (NSAID) commonly used for the reduction of mild to moderate pain, fever, inflammation and stiffness caused by conditions such as osteoarthritis, rheumatoid arthritis, psoriatic arthritis, gout, ankylosing spondylitis, injury (like fractures), menstrual cramps, tendonitis, bursitis, and the treatment of primary dysmenorrhea. Naproxen and naproxen sodium are marketed under various trade names including: Aleve, Anaprox, Naprogesic, Naprosyn, Naprelan.
Naproxen was first marketed as the prescription drug Naprosyn in 1976 and naproxen sodium was first marketed under the trade name Anaprox in 1980. It remains a prescription-only drug in much of the world. The U.S. Food and Drug Administration (FDA) approved the use of naproxen sodium as an over-the-counter (OTC) drug in 1991, where OTC preparations are sold under the trade name Aleve. In Australia, small packets of lower-strength preparations of naproxen sodium are Schedule 2 Pharmacy Medicines.
Structure and details
Naproxen is a member of the 2-arylpropionic acid (profen) family of NSAIDs. It is an odorless, white to off-white crystalline substance. It is lipid-soluble, practically insoluble in water with a low pH (below pH 4), while freely soluble in water at 6 pH and above. Naproxen has a melting point of 153 °C.
Adverse effects and warnings
Like other NSAIDs, naproxen is capable of producing disturbances in the gastrointestinal tract. Taking the medication with food may help to alleviate this most commonly reported adverse effect.
Also like other NSAIDs, naproxen can inhibit the excretion of sodium and lithium. Extreme care must be taken by those who use this drug along with lithium supplements.
Naproxen is also not recommended for use with NSAIDs of the salicylate family (drugs may reduce each other's effects), nor with anticoagulants (may increase risk of bleeding).
Certain preparations of Naproxen are not recommended for use in patients with hypertension, as they contain sodium, worsening the effects of hypertension.
In August 2006, the journal Birth Defects Research Part B published results in the September issue indicating that pregnant women who take NSAIDs including Naproxen in the first trimester run an increased risk of having a child with congenital birth defects, particularly heart anomalies.
In December 2004, the FDA issued a press release following the decision by the National Institutes of Health to halt a five-year study, called the Alzheimer's Disease Anti-Inflammatory Prevention Trial. That study aimed to test both Aleve and Celebrex as preventives for Alzheimer's disease. Preliminary information from the study showed naproxen elevated the risk of heart attack and stroke by 50%. The FDA advised patients taking over-the-counter naproxen products to:
- carefully follow the instructions on the label,
- avoid exceeding the recommended doses for naproxen (220 milligrams twice daily), and
- take naproxen for no longer than ten days unless a physician directs otherwise.
External links
- CID 1302 from PubChem
- EINECS number 244-838-7
- MedlinePlus Information on naproxen
- FDA Statement on Naproxen, released December 20, 2004
- Alzheimer's Disease Anti-Inflammatory Prevention Trial




 216.73.216.2
216.73.216.2 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 185.191.xxx.xx
185.191.xxx.xx
 Server Time:
Server Time: