 |
|
|
Indometacin
|
|
| Systematic (IUPAC) name | |
|
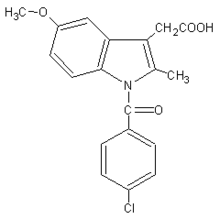
1-(4-chlorobenzoyl)-5-methoxy- 2-methyl-1-H-indole-3-acetic acid |
|
| Identifiers | |
| CAS number | 53-86-1 |
| ATC code | C01EB03 M01AB01, M02AA23, S01BC01 |
| PubChem | 3715 |
| DrugBank | APRD00109 |
| Chemical data | |
| Formula | C19H16NClO4 |
| Mol. weight | 357.79 g.mol-1 |
| Pharmacokinetic data | |
| Bioavailability | ~100% (oral), 80–90% (rectal) |
| Protein binding | 99% |
| Metabolism | Hepatic |
| Half life | 4.5 hours |
| Excretion | Renal 60%, faecal 33% |
| Therapeutic considerations | |
| Pregnancy cat. | C (Au), C/D (US) |
| Legal status | ℞ Prescription only |
| Routes | Oral, rectal, IV, topical |
Indometacin (INN) or Indomethacin (USAN and former BAN) is a non-steroidal anti-inflammatory drug commonly used to reduce fever, pain, stiffness, and swelling. It works by inhibiting the production of prostaglandins, molecules known to cause these symptoms. It is marketed under many trade names, including Indocin, Indocid, Indochron E-R, and Indocin-SR.
Contents |
Chemical properties
Indometacin is a methylated indole derivative and a member of the arylalkanoic acid class of NSAIDs, which includes diclofenac.
Indications
Clinical indications for indometacin include:
- ankylosing spondylitis
rheumatoid arthritis
arthritic gout
osteoarthritis
juvenile arthritis
psoriatic arthritis
Reiter's syndrome
Paget's disease of bone
Bartter syndrome
pseudogout
dysmenorrhea (menstrual cramps)
pericarditis
bursitis
tendinitis
nephrogenic diabetes insipidus (prostaglandin inhibits vasopressin's action in the kidney)
fever and pain associated with malignant diseases (tumors, bony metastases, lymphogranulomatosis)
Paroxysmal hemicrania, hemicrania continua and migraine
Indometacin has also been used clinically to delay premature labor, reduce amniotic fluid in polyhydramnios, and to treat patent ductus arteriosus.
Indometacin is a potent drug with many serious side effects and should not be considered an analgesic for minor aches and pains or fever. The drug is more potent than aspirin, but is not a better analgesic. In mild to moderate pain a standard oral dose of indometacin proved as effective as 600mg aspirin.
Contraindications
- concurrent peptic ulcer, or history of ulcer disease
allergy to indometacin, aspirin, or other NSAIDs
patients with nasal polyps reacting with an angioedema to other NSAIDs
children under 2 years of age (with the exception of neonates with patent ductus arteriosus)
severe preexisting renal and liver damage
caution : preexisting bone marrow damage (frequent blood cell counts are indicated)
caution : bleeding tendencies of unknown origin (indometacin inhibits platelet aggregation)
caution : Parkinson's disease, epilepsy, psychotic disorders (indometacin may worsen these conditions)
Mechanism of action
Indometacin is a nonselective inhibitor of cyclooxygenase (COX) 1 and 2, enzymes that participate in prostaglandin synthesis from arachidonic acid. Prostaglandins are hormone-like molecules normally found in the body, where they have a wide variety of effects, some of which lead to pain, fever, and inflammation.
Prostaglandins also cause uterine contractions in pregnant women. Indometacin is an effective tocolytic agent, able to delay premature labor by reducing uterine contractions through inhibition of PG synthesis in the uterus and possibly through calcium channel blockade.
Indometacin has two additional modes of actions with clinical importance:
- It inhibits motility of polymorphonuclear leucocytes, similar to colchicine.
- It uncouples oxidative phosphorylation in cartilaginous (and hepatic) mitochondria, like salicylates.
These additional effects account as well for the analgesic and the anti-inflammatory properties.
Indometacin readily crosses the placenta, and can reduce fetal urine production to treat polyhydramnios. It does so by reducing renal blood flow and increasing renal vascular resistance, possibly by enhancing the effects of vasopressin on the fetal kidneys.
Adverse effects
Since indometacin inhibits both COX-1 and COX-2, it inhibits the production of prostaglandins in the stomach and intestines which maintain the mucous lining of the gastrointestinal tract. Indometacin, therefore, like other nonselective COX inhibitors, can cause peptic ulcers. The ulcers can result in serious bleeding and/or perforation requiring hospitalization of the patient. Some even die from these complications. To reduce the possibility of peptic ulcers, indometacin should be prescribed at the lowest dosage needed to achieve a therapeutic effect, usually between 50–200 mg/day. It should always be taken with food. Nearly all patients benefit from an ulcer protective drug (e.g. highly dosed antacids, ranitidine 150mg at bedtime, or omeprazole 20mg at bedtime). Other common gastrointestinal complaints, including dyspepsia, heartburn and mild diarrhea are less serious and rarely require discontinuation of indometacin.
Many NSAIDs, but particularly indometacin, cause lithium retention by reducing its excretion by the kidneys. Thus indometacin users have an elevated risk of lithium toxicity. For patients taking lithium supplements (e.g. for treatment of depression or bipolar disorder), less toxic NSAIDs such as sulindac or aspirin, are preferred.
Indometacin also reduces plasma renin activity and aldosterone levels, and increases sodium and potassium retention. It also enhances the effects of vasopressin. Together these may lead to:
- edema (swelling due to fluid retention)
hyperkalemia (high potassium levels)
hypernatremia (high sodium levels)
hypertension (high blood pressure)
The drug may also cause elevations of serum creatinine and more serious renal damage such as acute renal failure, chronic nephritis and nephrotic syndrome. These conditions also often begin with edema and hyperkalema.
Additionally, indometacin quite often causes headache (10 to 20%), sometimes with vertigo and dizziness, hearing loss, tinnitus, blurred vision (with or without retinal damage) and worsens Parkinson's disease, epilepsy, and psychiatric disorders. Cases of life-threatening shock (including angioedema, sweating, severe hypotension and tachycardia as well as acute bronchospasm), severe or lethal hepatitis and severe bone marrow damage have all been reported. Skin reactions and photosensitivity are also possible side effects.
Due to its strong antipyretic activity indometacin may obscure the clinical course of serious infections.
The frequency and severity of side effects and the availability of better tolerated alternatives make indometacin today a drug of second choice. Its use in acute gout attacks and in dysmenorrhea is well-established because in these indications the duration of treatment is limited to a few days only, therefore serious side effects are not likely to occur.
Necessary Examinations during Longterm Treatment
Patients should undergo regular physical examination to detect edema and signs of central nervous side effects. Blood presssure checks will reveal development of hypertension. Periodic serum electrolyte (sodium, potassium, chloride) measurements, complete blood cell counts and assessment of liver enzymes as well as of creatinine (renal function) should be performed. This is particularly important if indometacin is given together with an ACE inhibitor or with potassium-sparing diuretics, because these combinations can lead to hyperkalemia and/or serious kidney failure. No examinations are necessary if only the topical preparations (spray or gel) are applied.
Animal Toxicity and Human Overdose
Indomethacin has a high acute toxicity both for animals (12 mg/kg in rats and 50 mg/kg in mice) and for humans. Exact human data does not exist, but some fatal human cases, particularly in children and adolescents, have been seen.
Generally, overdose in humans causes drowsiness, dizziness, severe headache, mental confusion, paraesthesia, numbness of limbs, nausea and vomiting. Severe gastrointestinal bleeding is also possible. Cerebral edema, and cardiac arrest with fatal outcome have been seen in children.
The treatment is symptomatic and largely the same as with diclofenac. However, the possibility of severe GI tract symptoms should be particularly noted.
The risk of overdose after exaggerated local treatment with gel or spray is very limited.
Usual Dosage Forms
- Tablets or Capsules 25 and 50mg
- Suppositories 50 and 100mg
- Modified-release Capsules 75mg
- Syrup (25mg/5ml)
- Injectable concentrate 50mg for i.m. injection
- Spray or Gel
- Patches containing 0.5% by weight
- 1% topical liquid
History
Indometacin was discovered in 1963 and it was first approved for use in the U.S. by the Food and Drug Administration in 1965. Its mechanism of action, along with several other NSAIDs that inhibit COX, was described in 1971.
References
- Lum G, Aisenbrey G, Dunn M, Berl T, Schrier R, McDonald K (Jan 1977). "In vivo effect of indomethacin to potentiate the renal medullary cyclic AMP response to vasopressin." (PDF or scanned copy). J Clin Invest 59 (1): 8-13. PMID 187624.
- Akbarpour F, Afrasiabi A, Vaziri N (1985). "Severe hyperkalemia caused by indomethacin and potassium supplementation.". South Med J 78 (6): 756-7. PMID 4002013.
- Ragheb M (Oct 1990). "The clinical significance of lithium-nonsteroidal anti-inflammatory drug interactions.". J Clin Psychopharmacol 10 (5): 350-4. PMID 2258452.
- Phelan K, Mosholder A, Lu S (Nov 2003). "Lithium interaction with the cyclooxygenase 2 inhibitors rofecoxib and celecoxib and other nonsteroidal anti-inflammatory drugs.". J Clin Psychiatry 64 (11): 1328-34. PMID 14658947.
- Hart F, Boardman P (Oct 1963). "Indomethacin: A new non-steroid anti-inflammatory agent.". Br Med J 5363: 965-70. PMID 14056924.
- Ferreira S, Moncada S, Vane J (Jun 23 1971). "Indomethacin and aspirin abolish prostaglandin release from the spleen.". Nat New Biol 231 (25): 237-9. PMID 5284362.
- Scherzer P, Wald H, Rubinger D, Popovtzer M (sep 1992). "Indomethacin and sodium retention in the rat: role of inhibition of prostaglandin E2 synthesis.". Clin Sci (Lond) 83 (3): 307-11. PMID 1327647.
External links
- Effects of Perinatal Indomethacin Treatment on Preterm Infants, academic dissertation (PDF)
- Indomethacin, from MedicineNet
- Indomethacin, from Drugs.com
- Indocin: Description, chemistry, ingredients, from RxList.com
- Japanese Indomethacin patches




 216.73.216.2
216.73.216.2 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.x
216.73.xxx.x
 Server Time:
Server Time: