 |
|
|
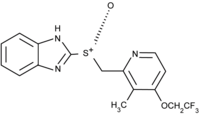
Lansoprazole
|
|
| Systematic (IUPAC) name | |
| 2-[(3-methyl-4-(2,2,2-trifluoroethoxy) pyridin-2-yl) methylsulfinyl] -1H-benzoimidazole | |
| Identifiers | |
| CAS number | 103577-45-3 |
| ATC code | A02BC03 |
| PubChem | 3883 |
| DrugBank | APRD00077 |
| Chemical data | |
| Formula | C16H14N3F3OS |
| Mol. weight | 369.363 g/mol |
| Pharmacokinetic data | |
| Bioavailability | 80% or more |
| Metabolism | Hepatic (CYP3A4- and CYP2C19-mediated) |
| Half life | 1 - 1.5 hours |
| Excretion | Renal and fecal |
| Therapeutic considerations | |
| Licence data | US |
| Pregnancy cat. | B3(AU) B(US) |
| Legal status | ℞ Prescription only |
| Routes | Oral, IV |
Lansoprazole (lan-SOE-pra-zole, INN) is a proton pump inhibitor which prevents the stomach from producing acid.
Contents |
Pharmacology
Lansoprazole is a proton pump inhibitor (PPI) similar to omeprazole. Lansoprazole's plasma elimination half-life is not proportional to the duration of the drug's effects (i.e. gastric acid suppression). The plasma elimination half-life is 1.5 hours or less, and the effects of the drug last for over 24 hours after it has been used for 5 days or more.
Indications
Lansoprazole is indicated for:
- Treatment of ulcers of the stomach and duodenum, and
NSAID-induced ulcers
Treatment of gastroesophageal reflux disease (GERD) (also known as acid reflux disease)
Treatment of Zollinger-Ellison Syndrome
Treatment of Barrett's esophagus
Adjunctive treatment of Helicobacter pylori infection, alongside antibiotics
Contraindications
- Absorption of lansoprazole is reduced by antacids.
- PPI’s reduce absorption of antifungals (itraconazole and ketoconazole) and possibly increase Digoxin in plasma
- Increases plasma concentrations of Cilostazol (risk of toxicity)
- Absorption of lansoprazole possibly reduced by:
- sucralfate
- ampicillin
- bisacodyl
delavirdine
fluvoxamine - iron salts
- theophylline
- voriconazole
aminophylline and theophylline
astemizole
Side effects
- Infrequent: dry mouth, insomnia, drowsiness, blurred vision, rash, pruritus
- Rarely and very rarely: taste disturbance, liver dysfunction, peripheral oedema, hypersensitivity reactions (including bronchospasm, urinary, angioedema, anaphylaxis), photosensitivity, fever, sweating, depression, interstitial nephritis, blood disorders (including leukopenia, leukocytosis, pancytopenia, thrombocytopenia), arthralgia, myalgia, skin reactions (including Stevens-Johnson syndrome, toxic epidermal necrolysis, bullous eruption)
- Increases the risk of gastric-intestinal infections by reducing gastric acidity.
- Severe: Gastro-intestinal disturbances (such as nausea, vomiting, abdominal pain, flatulence, diarrhea, constipation), headache, dizziness
Brand names
The drug is sold with the following brand names:
- Lansox® (Italy)
- Limpidex® (Italy)
- Prevacid® (U.S. and Canada)
- Takepron® (Japan)
- Zoton® (Italy)
Lansoprazole is also available as a generic drug in Italy.
External links
- Prevacid (manufacturer's website)
- Prevacid Pediatrics (manufacturer's website)
- Prevpac® (manufacturer's website)
- Lansoprazole (patient information)




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xxx
216.73.xxx.xxx
 Server Time:
Server Time: