 |
|
|
Esomeprazole
|
|
| Systematic (IUPAC) name | |
|
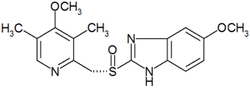
(S)-5-methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl) methylsulfinyl]-3H-benzoimidazole |
|
| Identifiers | |
| CAS number | 161796-78-7 |
| ATC code | A02BC05 |
| PubChem | 4594 |
| DrugBank | APRD00363 |
| Chemical data | |
| Formula | C17H19N3O3S |
| Mol. weight | 345.417 g/mol |
| Pharmacokinetic data | |
| Bioavailability | 50 to 90% |
| Metabolism | Hepatic (CYP2C19, CYP3A4) |
| Half life | 1–1.5 hours |
| Excretion | 80%
Renal 20% faecal |
| Therapeutic considerations | |
| Pregnancy cat. | B3(AU) |
| Legal status | S4(AU) POM(UK) |
| Routes | Oral, IV |
Esomeprazole (IPA: [ɛs oʊˈmɛprəzoʊl]) is a proton pump inhibitor (brand names Nexium®; Lucen®; Esopral® and Axagon® in Italy) used in the treatment of dyspepsia, peptic ulcer disease (PUD), gastroesophageal reflux disease (GORD/GERD) and Zollinger-Ellison syndrome. Esomeprazole is the S-enantiomer of omeprazole (marketed as Losec/Prilosec), and AstraZeneca claims improved efficacy of this single enantiomer product over the racemic mixture of omeprazole (see below).
Contents |
Pharmacology
Main article: Proton pump inhibitor
Esomeprazole is a proton pump inhibitor which reduces gastric acid secretion through inhibition of H+/K+-ATPase in gastric parietal cells. By inhibiting the functioning of this enzyme, the drug prevents formation of gastric acid.
Clinical use
Main article: Proton pump inhibitor
Use in Helicobacter pylori eradication
Esomeprazole is combined with the antibiotics clarithromycin and amoxicillin (or metronidazole in penicillin-hypersensitive patients) in the one week eradication triple therapy for Helicobacter pylori. Infection by H. pylori is the causative factor in the majority of peptic and duodenal ulcers.
Evidence of efficacy
AstraZeneca claims that esomeprazole provides improved efficacy, in terms of stomach acid control, over racemic omeprazole. Many health professionals have expressed the view that this improvement in efficacy is due to the increased dose of (es)omeprazole recommended for therapy rather than any superiority of esomeprazole per se.
The study usually cited by AstraZeneca to support its claims is of doubtful study power. Lind et al. (2004) compared esomeprazole to omeprazole in a study of only 36 GERD patients. It is noted that this study was financially supported by AstraZeneca itself.
An alternative rationale suggested for the use of esomeprazole was the reduction in interindividual variability in efficacy. There is, however, little evidence for even this advantage. (Somogyi et al., 2004)
Given the large difference in cost between esomeprazole and other proton pump inhibitors and the negligible advantages of esomeprazole, many doctors recommend cheaper alternatives, which in most cases work just as well.
Dosage forms
Esomeprazole is available as delayed-release capsules (containing esomeprazole magnesium) in strengths of 20 mg and 40 mg; and as a powder (esomeprazole sodium) for intravenous injection/infusion. Oral esomeprazole preparations are enteric-coated, due to the rapid degradation of the drug in the acidic conditions of the stomach. This is achieved by formulating capsules using the multiple-unit pellet system.
Multiple unit pellet system
Esomeprazole capsules are formulated as a "multiple unit pellet system" (MUPS). Essentially, the capsule consists of extremely small enteric-coated granules (pellets) of the esomeprazole formulation inside an outer shell. When the capsule is immersed in an aqueous solution, as happens when the capsule reaches the stomach, water enters the capsule by osmosis. The contents swell from water absorption causing the shell to burst, releasing the enteric-coated granules. For most patients, the multiple-unit pellet system is of no advantage over conventional enteric-coated preparations. Patients for which the formulation is of benefit include those requiring nasogastric tube feeding and those with difficulty swallowing (dysphagia).
The granules are manufactured in a fluid bed system with small sugar spheres as the starting material. The sugar spheres are sequentially spray-coated with a suspension containing esomeprazole, a protective layer to prevent degradation of the drug in manufacturing, an enteric coating and an outer layer to reduce granule aggregation. The granules are mixed with other inactive excipients and compressed into tablets. Finally, the tablets are film-coated to improve the stability and appearance of the preparation.
References
- Lind T., Rydberg L., Kyleback A., Jonsson A., Andersson T., Hasselgren T., Holmberg J., Rohss K. (2004). Esomeprazole provides improved acid control vs omeprazole in patients with symptoms of gastro-oesophageal reflux disease. Aliment Pharmacol Ther 14, 861-867.
- Somogyi A., Bochner F., Foster D. (2004). Inside the isomers: the tale of chiral switches. Aust Prescr 27, 47-49.
- Gladwell M., High Prices, New Yorker, Oct 2004. This article describes AstraZeneca's strategy for developing Nexium as a follow-on for Losec as the latter approached patent expiry.




 216.73.216.81
216.73.216.81 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xx
216.73.xxx.xx
 Server Time:
Server Time: