 |
|
|
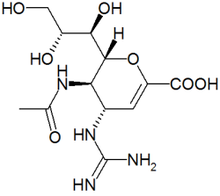
Zanamivir
|
|
| Systematic (IUPAC) name | |
|
5-acetamido-4-guanidino-6-(1,2,3-trihydroxypropyl)- 5,6-dihydro-4H-pyran-2-carboxylic acid |
|
| Identifiers | |
| CAS number | 139110-80-8 |
| ATC code | J05AH01 |
| PubChem | 60855 |
| DrugBank | APRD00378 |
| Chemical data | |
| Formula | C12H20N4O7 |
| Mol. weight | 332.31 g/mol |
| Pharmacokinetic data | |
| Bioavailability | 2% (oral) |
| Protein binding | <10% |
| Metabolism | Negligible |
| Half life | 2.5–5.1 hours |
| Excretion | Renal |
| Therapeutic considerations | |
| Pregnancy cat. | B1 (Au) |
| Legal status | S4 (Au), POM (UK), ℞-only (U.S.) |
| Routes | Inhalation |
Zanamivir (INN) (IPA: [zəˈnæmɪvir]) is a neuraminidase inhibitor used in the treatment of and prophylaxis of both Influenzavirus A and Influenzavirus B. Zanamivir was the first neuraminidase inhibitor commercially developed. It is currently marketed by GlaxoSmithKline under the trade name Relenza.
Contents |
Development
Zanamivir was developed in 1989 by scientists at the Australian biotechnology company Biota, working in conjunction with the CSIRO and the Victorian College of Pharmacy. The development was part of Biota's ongoing program to develop antiviral agents through rational drug design.
The strategy relied on the availability of the crystal structure of influenza neuraminidase which was achieved by x-ray crystallography. It was known as far back as 1974 that 2-deoxy-2,3-didehydro-N-acetylneuraminic acid (DANA), a sialic acid analogue, was an inhibitor of neuraminidase.[1] Using the crystal structure of neuraminidase and DANA as a starting point, the researchers employed a computer-aided process to attempt to design a molecule which was a better fit for (and therefore inhibited) the active site of neuraminidase. Zanamivir, a transition-state analogue inhibitor of neuraminidase, was the result.[2]
In 1990, zanamivir was licensed to Glaxo (now GlaxoSmithKline) for exclusive worldwide development and marketing. In 1999, the product was approved for marketing in the US and subsequently has been registered by GSK in a total of 70 countries.
Limitations
Whilst zanamivir proved to be a potent and effective inhibitor of influenza neuraminidase and inhibitor of influenza virus replication in vitro and in vivo, this didn't necessarily translate into a successful clinical treatment for influenza. In clinical trials it was found that zanamivir was able to reduce the time to symptom resolution by 1.5 days provided therapy was started within 48 hours of the onset of symptoms.
A further limitation concerns the poor oral bioavailability of zanamivir. This meant that oral dosing was impossible, limiting dosing to the parenteral routes. Zanamivir, therefore, is administered by inhalation - a route that was chosen for patient compliance with therapy. But even this route of administration is not acceptable to many in the community.
Commercial difficulties
Biota, being only a small company, was not able to bring the drug to market by itself. Consequently, it was licensed to Glaxo (now GlaxoSmithKline) to complete development and to market internationally as Relenza, delivered via Glaxo's proprietary, and some would say cumbersome, Diskhaler inhalation device. The license agreement entitled Biota to receive a 7% royalty on Glaxo's sales of Relenza.
A combination of factors has resulted in the limited commercial success of zanamivir (Relenza). The relatively small effect on the timecourse of influenza symptoms, the inhalation dosage form, a less-than-ideal device, and high expense make it a difficult product to market well. And although zanamivir was the first neuraminidase inhibitor to the market, it had only a few months lead over the second entrant, oseltamivir (Tamiflu), with an oral formulation much preferred by patients. Faced with this competition, GSK effectively abandoned the product.
When first marketed in 1999/00, Relenza captured close to 50% of the global market for neuraminidase inhibitors. But after the launch year, GSK cut virtually all promotion and other support for Relenza, allowing the product's sales and market share to slide in every major market over the following four years. By 2004 Relenza held only a 3% share of the estimated US$330 million global market.
In May 2004, Biota issued a writ against GSK for failing to support and promote Relenza. The writ claimed that GSK was in breach of several obligations:
- GSK restricted Relenza to its proprietary Diskhaler system, and did not adequately pursue alternative or improved inhalation systems.
- GSK withdrew support for crucial post-approval clinical studies designed to expand the product's use and market acceptance.
- After the launch year, GSK failed to properly launch Relenza in a number of countries where the product was registered, and allowed registrations to be stopped, cancelled or scheduled for cancellation.
- After the launch year, GSK withdrew promotion support for Relenza, allowing the sales and market share to decline in all key markets, even in those markets where there was no direct competition.
Developments from zanamivir
Zanamivir was the first of the neuraminidase inhibitors. Despite the limited commercial success of this drug, the work and strategies employed in the development of zanamivir were important first-steps in the development of further members of this class including oseltamivir and the candidate drug RWJ-270201 (Phase I trials). As a result more effective and potent treatments for influenza may be developed in the future.
References
- ^ Meindl P, Bodo G, Palese P, Schulman J, Tuppy H. Inhibition of neuraminidase activity by derivatives of 2-deoxy-2,3-dehydro-N-acetylneuraminic acid. Virology 1974;58(2):457-463. PMID 4362431
- ^ von Itzstein M, Wu W-Y, Kok GB, Pegg MS, Dyason JC, Jin B, et al. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature 1993;363(6428):418-423. PMID 8502295




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xxx
216.73.xxx.xxx
 Server Time:
Server Time: