 |
|
|
Oseltamivir
|
|
| Systematic (IUPAC) name | |
|
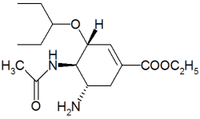
(3R,4R,5S)-4-acetylamino-5-amino- 3-(1-ethylpropoxy)-1-cyclohexene- 1-carboxylic acid ethyl ester |
|
| Identifiers | |
| CAS number | 196618-13-0 |
| ATC code | J05AH02 |
| PubChem | 65028 |
| DrugBank | APRD01148 |
| Chemical data | |
| Formula | C16H28N2O4 |
| Mol. weight | 312.4 g/mol |
| Pharmacokinetic data | |
| Bioavailability | 75% |
| Metabolism | hepatic, to GS4071 |
| Half life | 610 hours |
| Excretion | renal (GS4071) |
| Therapeutic considerations | |
| Pregnancy cat. | B1 (Australia), C (U.S.) |
| Legal status | Schedule 4 (Australia) POM (UK), ℞-only (U.S.) |
| Routes | oral |
Oseltamivir (INN) (IPA: [ɒsɛlˈtĉmɪvir]) is an antiviral drug that is used in the treatment and prophylaxis of both Influenzavirus A and Influenzavirus B. Like zanamivir, oseltamivir is a neuraminidase inhibitor. It acts as a transition-state analogue inhibitor of influenza neuraminidase, preventing new viruses from emerging from infected cells.
Oseltamivir was the first orally active neuraminidase inhibitor commercially developed. It is a prodrug, which is hydrolysed hepatically to the active metabolite, the free carboxylate of oseltamivir (GS4071). It was developed by Gilead Sciences and is currently marketed by Hoffmann-La Roche (Roche) under the trade name Tamiflu. It is generally available by prescription only.
With increasing fears about the potential for a new influenza pandemic, oseltamivir has received substantial media attention. Production capacity is limited, and governments (and even some private individuals) are stockpiling the drug.
Contents |
Clinical use
Indications and dosage
Oseltamivir is indicated for the treatment of infections due to influenza A and B virus in people at least one year of age, and prevention of influenza in people at least 1 year or older. The usual adult dosage for treatment of influenza is 75 mg twice daily for 5 days, beginning within 2 days of the appearance of symptoms and with decreased doses for children and patients with renal impairment. Oseltamivir may be given as a preventive measure either during a community outbreak or following close contact with an infected individual. Standard prophylactic dosage is 75 mg once daily for patients aged 13 and older, which has been shown to be safe and effective for up to six weeks. (Roche, 2005; Rossi, 2006)
Use in avian influenza
It has also been found that the standard recommended dose incompletely suppresses viral replication in at least some patients with H5N1 influenza, rendering therapy ineffective and increasing the risk of viral resistance (de Jong et al. 2005). Accordingly, it has been suggested that higher doses and longer durations of therapy should be used for treatment of patients with the H5N1 virus (de Jong et al. 2005, Ward et al. 2005). See Resistance section, below.
Chokephaibulkit et al recommend the use of oseltamivir in pediatric patients, based on experience with one patient.
After following WHO protocols in treating 41 victims of the H5N1 bird flu virus (19% of the world-wide cases of bird flu reported to date), Nguyen Tuong Van, MD, who runs the intensive care unit of the Center for Tropical Diseases in Hanoi, Vietnam concluded that Tamiflu, the drug most widely stockpiled around the world to combat a potential bird flu pandemic, is "useless." According to this article, the WHO confirmed Van's experience stating that Tamiflu has not been "widely successful in human patients", but speculated the drug has not been administered until late in the disease in many Asian countries.
Co-administration with probenecid
It has been suggested that co-administration of oseltamivir with probenecid could extend the limited supply of oseltamivir. Probenecid reduces renal excretion of the active metabolite of oseltamivir. One study showed that 500 mg of probenicid given every six hours doubled both the peak plasma concentration (Cmax) and the half-life of oseltamivir, increasing overall systemic exposure (AUC) by 2.5-fold. (Hill et al., 2002) Although the evidence for this interaction comes from a study by Roche, it was publicised only in October 2005 by a doctor who had reviewed the data (Butler, 2005). Probenecid was used in similar fashion during World War II to extend limited supplies of penicillin, and is still currently used to increase penicillin concentrations in serious infections.
Dosage forms
Oseltamivir is marketed by Roche under the trade name Tamiflu, as capsules (containing oseltamivir phosphate 98.5 mg equivalent to oseltamivir 75 mg) and as a powder for oral suspension (oseltamivir phosphate equivalent to oseltamivir 12 mg/mL).
Adverse effects
Common adverse drug reactions (ADRs) associated with oseltamivir therapy include: nausea, vomiting, diarrhea, abdominal pain, and headache. Rare ADRs include: hepatitis and elevated liver enzymes, rash, allergic reactions including anaphylaxis, and Stevens-Johnson syndrome. (Rossi, 2006)
Various other ADRs have been reported in postmarketing surveillance including: toxic epidermal necrolysis, cardiac arrhythmia, seizure, confusion, aggravation of diabetes, and haemorrhagic colitis.
Neurological effects
In May 2004, the safety division of Japan's health ministry ordered changes to the literature accompanying oseltamivir to add neurological and psychological disorders as possible adverse effects, including: impaired consciousness, abnormal behavior, and hallucinations. Various cases of psychological disorders were associated with oseltamivir therapy between 20002004, including several deaths.
On 2005-11-18 the United States Food and Drug Administration (FDA) issued a report regarding the paediatric safety of oseltamivir, which stated that there was insufficient evidence to claim a causal link between oseltamivir use and the deaths of 12 Japanese children (only two from neurological problems). However, it was recommended that a warning was added to the Product Information regarding rashes associated with oseltamivir therapy (Pediatric Advisory Committee, 2005).
In November 2006, reports of bizarre behavior in Japanese children, including three deaths from falls, resulted in the FDA amending the warning label to include possible side effects of delirium, hallucinations, or other related behavior.[1]
Mode of action
Oseltamivir is a neuraminidase inhibitor. By blocking the activity of the neuraminidase, Oseltamivir prevents new viral particles from being released by infected cells.
Resistance
As with other antivirals, resistance to the agent was expected with widespread use of oseltamivir, though the emergence of resistant viruses was expected to be less frequent than with amantadine or rimantadine. The resistance rate reported during clinical trials up to July 2004 was 0.33% in adults, 4.0% in children, and 1.26% overall. Mutations conferring resistance are single amino acid residue substitutions in the neuraminidase enzyme (Ward et al., 2005).
Mutant H3N2 influenza A virus isolates resistant to oseltamivir were found in 18% of a group of 50 Japanese children treated with oseltamivir (Kiso et al., 2004). This rate was similar to another study where resistant isolates of H1N1 influenza virus were found in 16.3% of another cohort of Japanese children (Ward et al., 2005). Several explanations were proposed by the authors of the studies for the higher-than-expected resistance rate detected. First, children typically have a longer infection period, giving a longer time for resistance to develop. Second, Kiso et al. (2004) claim to have used more rigorous detection techniques than previous studies. Third, the dosage regimen in Japan is different from that of other nations, and some children may have been given a suboptimal dosage of oseltamivir.
High-level resistance has been detected in one girl suffering from H5N1 avian influenza in Vietnam. She was being treated with oseltamivir at time of detection (Le et al., 2005; World Health Organization, 2005).
de Jong et al. (2005) describe resistance development in two more Vietnamese patients suffering from H5N1, and compare their cases with six others. They suggest that the emergence of a resistant strain may be associated with a patient's clinical deterioration. They also note that the recommended dosage of oseltamivir does not always completely suppress viral replication, a situation that could favor the emergence of resistant strains. Moscona (2005) gives a good overview of the resistance issue, and says that personal stockpiles of Tamiflu could lead to under-dosage and thus the emergence of resistant strains of H5N1.
Resistance is of concern in the scenario of an influenza pandemic (Wong and Yuen 2005), and may be more likely to develop in avian influenza than seasonal influenza due to the potentially longer duration of infection by novel viruses. Kiso et al. (2004) suggest that "a higher prevalence of resistant viruses should be expected" during a pandemic.
The genetic sequence for the neuraminidase enzyme is highly conserved across virus strains. This means that there are relatively few variations, and there is also evidence that variations that do occur tend to be less "fit." Thus, mutations that convey resistance to oseltamivir may also tend to cripple the virus by giving it an otherwise less-functional enzyme. The lack of variation in neuraminidase gives two advantages to oseltamivir and zanamivir, the drugs that target that enzyme. First, these drugs work on a broader spectrum of influenza strains. Second, the development of a robust, resistant virus strain appears to be less likely (Ward et al., 2005). It is worth noting that the oseltamivir-resistant strains detected by Kiso et al. (2004) all appeared within individual children after treatment with oseltamivir the children did not catch the resistant strains in human-to-human or bird-to-human transmission.
Pandemic fears
Oseltamivir was widely used during the H5N1 avian influenza epidemic in Southeast Asia in 2005. In response to the epidemic, various governments including those of the United Kingdom, Canada, United States and Australia stockpiled quantities of oseltamivir in preparation for a possible pandemic. Though large, the quantities stockpiled would not have been sufficient to protect the entire population of these countries.
In October 2005, the Indian drug company Cipla announced their plan to begin manufacture of generic oseltamivir without license from Roche. Most patent laws allow governments to authorise supply from generic companies, subject to remuneration to patent owners to address public health problems, including emergencies, although Roche has announced its intention to remain the sole supplier of the drug. Cipla argues that it can legally sell oseltamivir to India and 49 other developing countries, possibly as early as January 2006. Also in October, it was announced that Roche was in discussions with four generic drug manufacturers about the possibility of issuing sublicenses to increase production.
In late October 2005, Roche announced that it was suspending shipments to pharmacies in the United States and Canada until the North American seasonal flu outbreak began, to address concerns about private stockpiling and to preserve supplies for seasonal influenza. It said that, when distribution resumes in Canada, the remaining available drug will be saved for use in high-risk settings like long-term care facilities and hospitals. [1][2][3] Sales were suspended in Hong Kong as well, and on November 8, also in China. Roche said it would instead send all supplies to China's health ministry[4].
On November 9, 2005, Vietnam became the first country to be granted permission by Roche to produce a generic version of oseltamivir[5]. The week before, Thai authorities said they would begin producing generic oseltamivir, claiming that Roche had not patented Tamiflu in Thailand[6]. The first Thai generic oseltamivir was produced in February 2006 and are to be available to the public in July 2006[7].
In December 2005, Roche also signed a sublicense for complete oseltamivir production with China's Shanghai Pharmaceuticals, and by March 2006 a sublicense had also been granted to India's Hetero [8][9]. In June 2006, the Chinese government gave Shanghai Pharmaceuticals permission to proceed, based upon tests of the domestic production. The company said it planned to market the drug by the end of the month.[10][11]
In late May 2006, the World Health Organization asked Roche to be ready to ship an emergency stockpile of oseltamivir to Indonesia if needed. The alert was in response to suspected human-to-human transmission within a family and was planned to last for two weeks [12][13].
U.S. Government policy and oseltamivir
In November, 2005, U.S. president George W. Bush requested that Congress fund $7.1 billion in emergency spending for flu pandemic preparedness (the Senate had already passed an $8.1 billion bill)[14]. Bush's plan included $1.4 billion for government purchases of antiviral drugs[15].
Some commentators (e.g., [16]) question the motives of the U.S. government's endorsement and planned purchase of oseltamivir, noting Secretary of Defense Donald Rumsfeld's close ties to Gilead Sciences, rightsholder to the oseltamivir patent. Rumsfeld is a former chairman of Gilead, and federal disclosure forms indicate that he owns between USD$5 million and USD$25 million in Gilead stock (Schwartz 2005 [17]). The rise in Gilead's share prices from USD$35 to USD$57 per share will have added between USD$2.5 million to USD$15.5 million to Rumsfeld's net worth.
On the other hand, at least one Democratic Senator has criticized Bush for not planning to buy enough antiviral drugs [18].
Personal stockpiling of oseltamivir
The short supply of oseltamivir has prompted some individuals to stockpile the drug. Several American states, including Massachusetts and Colorado, have issued advisories strongly discouraging this practice.
One argument against individual stockpiling is that limited drugs should be kept for more strategic or ethical deployment, that is, to hard-hit areas, to people in critical roles (e.g., healthcare and government workers), to people vulnerable to seasonal flu, or to people who actually have come down with avian influenza. Ethical arguments are sometimes made: Why should affluent people (or nations) have preferred access to antiviral medications? Illegal importation may divert the drug from poorer countries where the risk of avian influenza is actually higher.
In the New England Journal of Medicine, Moscona (2005) argues that the use of personal stockpiles of oseltamivir could result in the administration of low dosages, allowing for the development of drug-resistant virus strains. Many stockpilers will only have ten 75 mg pills (the current recommended dosage for oseltamivir), but this may be insufficient for the treatment of H5N1. (de Jong et al., 2005)
Another argument is that it would be difficult for home users to determine whether illegally-imported Tamiflu is counterfeit. This is genuinely a potential problem, but, in the face of a shortage, some individuals may be willing to face such a risk. In December 2005, 53 packages of counterfeit Tamiflu tablets were intercepted by the US Customs Service in South San Francisco. The packages were labeled "Generic Tamiflu". Roche officials know of only one instance of counterfeit Tamiflu appearing outside of the United States: incorrectly-labelled tablets found in Holland, which contained only Vitamin C and lactose. However, sophisticated criminals could produce convincing fake packaging in the future. [19][20]
A fourth purported problem is that the H5N1 virus can be reliably diagnosed only in a small number of labs around the world; therefore, there is no way for home users to know whether flu-like symptoms are the result of avian flu or a more benign ailment. This argument lacks face validity, since treatment must begin before such tests results would be available anyway.
Veterinary use
Oseltamivir appears to be active against canine parvovirus, feline panleukopenia, the canine respiratory complex known as "kennel cough," and the emerging disease dubbed "canine flu", an equine virus that began affecting dogs in 2005. Veterinary investigation of its use for canine parvo [21] and canine flu [22]is ongoing, but many shelters and rescue groups have reported great success employing oseltamivir in the early stages of these illnesses.
Chemical synthesis
Commercial syntheses
The current production method includes two reaction steps with potentially hazardous azides. A reported azide-free Roche synthesis of tamiflu is summarised graphically below:
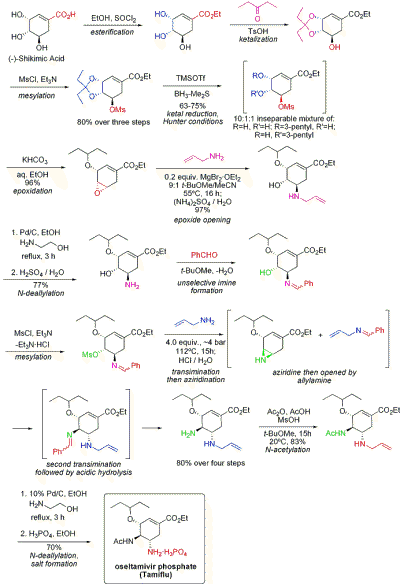
The synthesis commences from naturally available (−)-shikimic acid. The 3,4-pentylidene acetal mesylate is prepared in three steps: esterification with ethanol and thionyl chloride; ketalization with para-toluenesulfonic acid and 3-pentanone; and mesylation with triethylamine and methanesulfonyl chloride. Reductive opening of the ketal under modified Hunter conditions (JOC 1993, 58, 6756) in dichloromethane yields an inseparable mixture of isomeric mesylates. The corresponding epoxide is formed under basic conditions with potassium bicarbonate. Using the inexpensive Lewis acid magnesium bromide diethyl etherate (commonly prepared fresh by the addition of magnesium turnings to 1,2-dibromoethane in benzene:diethyl ether), the epoxide is opened with allyl amine to yield the corresponding 1,2-amino alcohol. The water-immiscible solvents methyl tert-butyl ether and acetonitrile are used to simplify the workup procedure, which involved stirring with 1 M aqueous ammonium sulfate. Reduction on palladium, promoted by ethanolamine, followed by acidic workup yielded the deprotected 1,2-aminoalcohol. The aminoalcohol was converted directly to the corresponding allyl-diamine in an interesting cascade sequence that commences with the unselective imination of benzaldehyde with azeotropic water removal in methyl tert-butyl ether. Mesylation, followed by removal of the solid byproduct triethylamine hydrochloride, results in an intermediate that was poised to undergo aziridination upon transimination with another equivalent of allylamine. With the librated methanesulfonic acid, the aziridine opens cleanly to yield a diamine that immediately undergoes a second transimination. Acidic hydrolysis then removed the imine. Selective acylation with acetic anhydride (under buffered conditions, the 5-amino group is protonated owing to a considerable difference in pKa, 4.2 vs 7.9, preventing acetylation) yields the desired N-acetylated product in crystalline form upon extractive workup. Finally, deallylation as above, yielded the freebase of oseltamivir, which was converted to the desired oseltamivir phosphate by treatment with phosphoric acid. The final product is obtained in high purity (99.7%) and an overall yield of 17-22% from (−)-shikimic acid. It is noted that the synthesis avoids the use of potentially explosive azide reagents and intermediates; however, the synthesis actually used by Roche uses azides. Roche has other routes to oseltamivir that do not involve the use of (−)-shikimic acid as a chiral pool starting material, such as a Diels-Alder route involving furan and ethyl acrylate or an isophthalic acid route, which involves catalytic hydrogenation and enzymatic desymmetrization.
2006 Corey synthesis
In 2006 the group of E.J. Corey published a novel route also bypassing shikimic acid starting from butadiene and acrylic acid. The inventors choose not to patent this procedure which is described below. Strictly speaking the synthesis is a formal total synthesis, as the final product to be characterized was the BOC-derivative (14 in the scheme below).
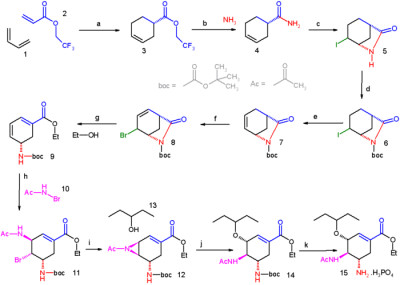
Butadiene 1 reacts in an asymmetric Diels-Alder reaction with the esterfication product of acrylic acid and 2,2,2-Trifluoroethanol 2 catalysed by the CBS catalyst. The ester 3 is converted into an amide in 4 by reaction with ammonia and the next step to lactam 5 is an iodolactamization with iodine initiated by trimethylsilyltriflate. The amide group is fitted with a BOC protective group by reaction with Boc anhydride in 6 and the iodine substituent is removed in an elimination reaction with DBU to the alkene 7. Bromine is introduced in 8 by an allylic bromination with NBS and the amide group is cleaved with ethanol and caesium carbonate accompanied by elimination of bromide to the diene ethyl ester 9. The newly formed double bond is functionalized with N-bromoacetamide 10 catalyzed with Tin(IV) bromide with complete control of stereochemistry. In the next step the bromine atom in 11 is displaced by the nitrogen atom in the amide group with the strong base KHMDS to the aziridine 12 which in turn is opened by reaction with 3-pentanol 13 to the ether 14. In the final step the BOC group is removed with phosphoric acid and the oseltamivir phosphate 15 is formed.
Production shortage/shikimic acid
In early 2005, Roche announced a production shortage. (See Pandemic Fears, below). According to Roche, the major bottleneck in oseltamivir production is the availability of shikimic acid, which cannot be synthesised economically and is only effectively isolated from Chinese star anise, an ancient cooking spice. Although most autotrophic organisms produce shikimic acid, the isolation yield is low. A shortage of star anise is one of the key reasons why there is a worldwide shortage of Tamiflu (as at 2005). Star anise is grown in four provinces in China and harvested between March and May. It is also produced in Lang Son province, Vietnam. The shikimic acid is extracted from the seeds in a ten-stage manufacturing process. Thirteen grams of star anise make 1.3 grams of shikimic acid, which can be made into 10 oseltamivir 75 mg capsules. Ninety percent of the harvest is already used by Roche in making oseltamivir.
Some academic experts and other drug companies are disputing the difficulty of producing shikimic acid by means other than star anise extraction. An alternative method for production of the acid involves fermentation of genetically-modified bacteria. Other potential sources of shikimic acid include the ginkgo tree. In addition, quinic acid, derived from the bark of the cinchona tree of the Democratic Republic of the Congo, is a potential alternative base material for the production of oseltamivir.
However, as is clear by the multistep synthesis shown above, although the major bottleneck for Roche may be the availability of shikimic acid, production of oseltamivir is very involved. Increasing production volume (by Roche or others) would require construction of extensive new facilities (which may not be amenable to scaleup and, even if identical on paper, may not necessarily produce acceptable yields), and even if current facilities could handle a larger feedstock quantity, there would be a delay in production as the material makes it down the pipeline (~6 months or so).
In March 2006, Roche announced that it was making utilizing the resources of 15 external contractors in 9 countries, allowing production to expand to "as much as 400 million doses annually by the end of this year" [23].
Canadian generic drug company Apotex is attempting to modify Oseletamivir to use a synthetic alternative to shikimic acid.
References
- ^ "Flu Drug Tamiflu May Cause Odd Behavior in Children", Forbes, 2006-11-13.
- Butler D. Wartime tactic doubles power of scarce bird-flu drug [News article]. Nature 2005;438(7064):6. (Accessed on November 2, 2005, at http://www.nature.com/nature/journal/v438/n7064/full/438006a.html)
- de Jong MD, Thanh, TT, Khanh, TH, Hien, VM, Smith, GJD, Chau, NV, et al. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med 2005;353(25):2667-2672. PMID 16371632 (full text)
- Hill G, Cihlar T, Oo C, Ho ES, Prior K, Wiltshire H, Barrett J, Liu B, Ward P. The anti-influenza drug oseltamivir exhibits low potential to induce pharmacokinetic drug interactions via renal secretion--correlation of in vivo and in vitro studies. Drug Metabolism and Disposition 2002;30(1):13-19. (Online at: http://dmd.aspetjournals.org/cgi/content/abstract/30/1/13)
- Kiso M, Mitamura K, Sakai-Tagawa Y, Shiraishi K, Kawakami C, Kimura K, et al. Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet 2004;364(9436):759-65. PMID 15337401
- Le Q M, Kiso M, Someya K, Sakai Y T, Nguyen T H, Nguyen K H L, Pham N D, Ngyen H H, Yamada S, Muramoto Y, Horimoto T, Takada A, Goto H, Suzuki T, Suzuki Y, Kawaoka Y. Avian flu: Isolation of drug-resistant H5N1 virus. Nature 2005;437(7062):1108.
- Moscona, Anne. Oseltamivir Resistance - Disabling Our Influenza Defenses [Perspective]. New England Journal of Medicine 2005;353(25):2633-2636.
- Pediatric Advisory Committee. Pediatric safety update for Tamiflu. Rockville (MD): U.S. Food and Drug Administration; 2005.
- Pollack, Andrew. Is Bird Flu Drug Really So Vexing? Debating the Difficulty of Tamiflu [News article]. The New York Times (Accessed on November 5, 2005 at http://www.nytimes.com/2005/11/05/business/05tamiflu.html)
- Roche Laboratories, Inc. Tamiflu product information. Last updated Dec. 2005. (Accessed on 20 February, 2006 at http://www.rocheusa.com/products/tamiflu/pi.pdf) prescribing information document from Roche
- Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006.
- Schwartz, Nelson . Oct 31, 2005. Rumsfeld's growing stake in Tamiflu: Defense Secretary, ex-chairman of flu treatment rights holder, sees portfolio value growing. Fortune (Accessed on Nov 28, 2005 at http://money.cnn.com/2005/10/31/news/newsmakers/fortune_rumsfeld/?cnn=yes)
- Wong, Samson S.Y. and Yuen, Kwok-yung. Avian influenza virus infections in humans. Chest 2006; 129(1):156-168.
- Ward P, Small I, Smith J, Suter P, Dutkowski R. Oseltamivir (Tamiflu) and its potential for use in the event of an influenza pandemic. J Antimicrob Chemother 2005;55(Suppl 1): i5-i21. PMID 15709056
- World Health Organization. WHO inter-country-consultation: influenza A/H5N1 in humans in Asia: Manila, Philippines, 6-7 May 2005. (Accessed October 12, 2005, at http://www.who.int/csr/resources/publications/influenza/WHO_CDS_CSR_GIP_2005_7/en/.)
- J. Org. Chem. 1998, 63, 4545-4550. Synthesis of Tamiflu.
- J. Org. Chem. 2001, 66, 2044-2051. Synthesis of Tamiflu.
- Chimia 2004, 58, 621.
- A Short Enantioselective Pathway for the Synthesis of the Anti-Influenza Neuramidase Inhibitor Oseltamivir from 1,3-Butadiene and Acrylic Acid Ying-Yeung Yeung, Sungwoo Hong, and E. J. Corey J. Am. Chem. Soc.; 2006; ASAP Web Release Date: 25-Apr-2006; (Communication) Abstract
See also
- Zanamivir - another neuraminidase inhibitor
External links
- www.tamiflu.com Roche's page on Tamiflu (oseltamivir)
- MedlinePlus Drug Information: oseltamivir (systemic) information from USP DI Advice for the Patient
- Pharmasquare Tamiflu Mode of Action Flash animation showing the mode of action of oseltamivir
- FDA information page on oseltamivir
- Flu Drugs FAQ U.S. National Institute of Allergy and Infectious Diseases
- Journal Response Oseltamivir abstracts of recent oseltamivir research




 216.73.216.81
216.73.216.81 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xx
216.73.xxx.xx
 Server Time:
Server Time: