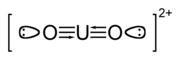

The uranyl ion is the dipositive cation UO22+, which forms salts with acids. In this ion, uranium is in its +6 oxidation state. The other common oxidation state of uranium is uranium(IV), called uranous. The uranyl ion is the most common species encountered in the aqueous chemistry of uranium. Solid uranyl compounds are often colored red, yellow, orange or green. Like all uranium compounds, uranyl compounds are toxic. The toxicity of soluble uranyl salts is higher due to their faster incorporation into tissues.
Contents |
Examples
Examples of uranyl compounds include:
- Uranium trioxide, UO3
- Uranyl acetate, UO2(C2H3O2)2
- Uranyl ammonium carbonate, UO2CO3·2(NH4)2CO3
- Uranyl carbonate, UO2CO3
- Uranyl chloride, UO2Cl2
- Uranyl hydroxide, UO2(OH)2 or (UO2)2(OH)2 also in aqueous
- Uranyl nitrate, UO2(NO3)2
- Uranyl sulfate, UO2SO4
- Uranyl zinc acetate, ZnUO2(CH3COO)4
Minerals
Such minerals occur in oxidised portions of uranium ore deposits. Common uranyl minerals include tyuyamunite (Ca(UO2)2V2O8·8H2O), autunite (Ca(UO2)2(PO4)2·8-12H2O), torbernite (Cu(UO2)2 (PO4)·8-12H2O) and uranophane (H3O)2Ca (UO2)2(SiO4)·3H2O) (Hutchinson and Blackwell, 1984). Uranyl minerals, which contain uranium(VI) can help show the genesis of uranium deposits and the water-rock interactions that occur in uranium-rich mineral seams.
Chemistry
Uranium chemistry has traditionally revolved around the aqueous chemistry of the uranyl ion, and related molecular species. One important use of this chemistry is for preparation of uranium dioxide ceramic pellets that are used as the fuel in light water nuclear reactors. Often the fuel materials start to break down chemically before the uranium is completely spent, and this too is an active area of investigation, as many of the corrosion products are of the uranyl group.
Structure
The geometry of the uranyl ion has been the subject of much debate. The close approach of two oxygen atoms to uranium, with each linear O-U-O bond from 1.7 to 1.9 Å, prevents the close approach of a third or more. d-p and f-p bonding have been suggested to explain the short U-O bonds.[1]
Health and environmental issues
Uranyl nitrate is an oxidizing and highly toxic compound and should not be ingested; it causes severe renal insufficiency and acute tubular necrosis and is a lymphocyte mitogen.
Target organs include the kidneys, liver, lungs and brain. Uranyl ion accumulation in tissues including gonocytes[2] produces congenital disorders, and in white blood cells causes immune system damage.[3] Uranyl compounds are also neurotoxins.
Combustion of uranium
Aerial oxidation of any uranium compound eventually results in the formation of a uranyl compound.[1] Uranyl ion contamination has been found on and around depleted uranium targets.[4]
References
- ^ a b c Cotton, S (1991). Lanthanides and Actinides. New York: Oxford University Press, 128.
- ^ Arfsten DP, Still KR, Ritchie GD (2001). "A review of the effects of uranium and depleted uranium exposure on reproduction and fetal development". Toxicology and Industrial Health 17: 180-191. DOI:10.1191/0748233701th111oa.
- ^ Schröder H, Heimers A, Frentzel-Beyme R, Schott A, Hoffman W (2003). "Chromosome Aberration Analysis in Peripheral Lymphocytes of Gulf War and Balkans War Veterans". Radiation Protection Dosimetry 103: 211-219.
- ^ Salbu B, Janssens K, Linda OC, Proost K, Gijsels L, Danesic PR (2004). "Oxidation states of uranium in depleted uranium particles from Kuwait". Journal of Environmental Radioactivity 78: 125-135. DOI:10.1016/j.jenvrad.2004.04.001.




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xxx
216.73.xxx.xxx
 Server Time:
Server Time: