 |
|
|
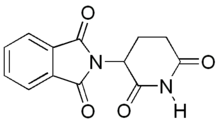
Thalidomide
|
|
| Systematic (IUPAC) name | |
| 2-(2,6-dioxo-3-piperidyl)isoindole-1,3-dione | |
| Identifiers | |
| CAS number | 50-35-1 |
| ATC code | L04AX02 |
| PubChem | 5426 |
| DrugBank | APRD01251 |
| Chemical data | |
| Formula | C13H10N2O4 |
| Mol. weight | 258.23 g/mol |
| Pharmacokinetic data | |
| Protein binding | 55% and 66% for the (+)R and (–)S enantiomers, respectively |
| Half life | mean ranges from approximately 5 to 7 hours following a single dose; not altered with multiple doses |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. | X(AU) X(US) |
| Legal status | ℞ Prescription only |
| Routes | oral |
|
Thalidomide is a sedative, hypnotic, and anti-inflammatory medication. It was sold from 1957 to 1961 in almost fifty countries under at least forty names, including Distaval, Talimol, Nibrol, Sedimide, Quietoplex, Contergan, Neurosedyn, and Softenon. Thalidomide was chiefly sold and prescribed during the late 1950s and 1960s to pregnant women, as an antiemetic to combat morning sickness and as an aid to help them sleep. Unfortunately, inadequate tests were performed to assess the drug's safety, with catastrophic results for the children of women who had taken thalidomide during their pregnancies.
From 1956 to 1962, approximately 10,000 children were born with severe malformities, including phocomelia, because their mothers had taken thalidomide during pregnancy.[1] In 1962, in reaction to the tragedy, the United States Congress enacted laws requiring tests for safety during pregnancy before a drug can receive approval for sale in the U.S.[2] Other countries enacted similar legislation, and thalidomide was not prescribed or sold for decades.
Researchers, however, continued to work with the drug. Soon after its banishment, a doctor discovered anti-inflammatory effects of thalidomide and began to look for uses of the medication despite its teratogenic effects. He found that patients with erythema nodosum leprosum, a painful skin condition associated with leprosy, experienced relief of their pain by taking thalidomide. Currently, there are studies underway to determine the drug's effects on arachnoiditis, Crohn's disease, and several types of cancers. However, physicians and patients alike must go through a special process to prescribe and receive thalidomide, to ensure no more children are born with birth defects traceable to the medication.
History
Discovery and introduction
A small German pharmaceutical company, Chemie Grünenthal, synthesized thalidomide in West Germany in 1953 while searching for an inexpensive method of manufacturing antibiotics from peptides. By heating phthaloyisoglutamine, the company's chief researcher produced phthalimidoglutarimide, which they soon labeled 'thalidomide.' Chemie Grünenthal patented the molecule and began searching for a disease thalidomide could cure.[3]
The Grünenthal scientists could not find any antibiotic activity, or any other encouraging effects, in mice and rats. However, they saw that the new chemical seemed to be harmless; outrageously high doses did not kill rodents, rabbits, cats, or dogs. In addition, the animals showed no other side effects. The research team began to describe thalidomide as "nontoxic," and Grünenthal began to consider the lucrative prospects of their new find. Notably, although no sedative or tranquilizing effects were observed in animals, Grünenthal management considered "a nonlethal sedative would have enormous market potential." [3]
With only these animal tests, no clinical trial plans, and no scientific rationale, Grünenthal began distributing free samples of thalidomide to doctors in Switzerland and West Germany in 1955. It was first recommended for the prevention of seizures in patients with epilepsy; although no anticonvulsant effect was found, patients reported experiencing a deep sleep. Other patients said they felt calming and soothing effects. Some reported side effects, but they were not believed to be serious.[3] One author later said that "Thalidomide was introduced by the method of Russian Roulette. Practically nothing was known about the drug at the time of its marketing."[4]
Thalidomide could not be sold in West Germany until its effects on animals were documented – usually effects of a drug are demonstrated on animals before the drug is administered to humans, but thalidomide was not tested that way. The sedative effects had not been seen in animals, so the Grünenthal scientists came up with a "jiggle cage" to measure the movements of mice to see if treated mice "jiggled" the cage less than non-treated mice. Grünenthal also pointed out that their "powerful hypnotic drug was completely safe.[3]
An employee of Chemie Grünenthal brought home samples of the new drug for his pregnant wife, and ten months before thalidomide was put on the market in Germany, on Christmas Day in 1956, their child was born – without ears. Years later, the father learned that his daughter was the first living victim of the epidemic of thalidomide-induced infant malformations and deaths.[3]
The company began selling the drug over the counter in Germany in October 1957, under the brand name Contergan. The company claimed that "Even a determined suicide could not take enough Contergan to cause death" and "accidental overdoses by children would be unheard of with this drug." Soon the drug was being sold in 46 countries under "at least 37 names,"[3] without any additional independent testing, and was the drug of choice for pregnant women with morning sickness.[5] Not one of those statements turned out to be true.
Frances Kelsey
By 1960, Grünenthal was selling thalidomide tablets all over the world – except in the United States. A Cincinnati, Ohio company called Richardson-Merrell tried to change that in September 1960, when it applied for Food and Drug Administration (FDA) approval to sell thalidomide in the United States under the brand name of Kevadon. The company wanted to begin sales of Kevadon in early 1961. This approval was not expected to be controversial, and the case was given to the agency's newest reviewer, Frances Oldham Kelsey, who had joined the FDA only one month before.
At the time, the prevailing US law was the 1938 Federal Food, Drug, and Cosmetic Act, which required proof of safety be sent to the FDA before a medication could be approved for sale in the United States. The law did not require demonstration of efficacy for approval. It also allowed "investigational" or "experimental" use of a drug while approval for its sale was being sought, meaning that a medication could be widely distributed before it was approved.[6] The law gave the FDA 60 days to review a drug application. If the FDA reviewer told a drug company that its application for a particular medication was incomplete, it was considered withdrawn and the company would have to submit more data when it resubmitted the application. With each resubmission, the 60 days started all over again.[1]
This was Kelsey's first drug review assignment for the FDA. She was interested in fetal safety because in the 1940s she had studied quinine, including its effects in pregnancy, while she was part of a team that was trying to find a synthetic cure for malaria. In particular, she recalled a study she conducted on rabbits at the University of Chicago. In that study, she noted that adult rabbits metabolized quinine rapidly, but pregnant rabbits were less able to metabolize it and embryonic rabbits could not metabolize it at all. In addition, contrary to the prevailing theories of the time, Kelsey found that the drug passed through the placental barrier between mother and fetus.[5] Recalling her work with quinine, Kelsey thought that perhaps thalidomide acted the same way – if so, thalidomide might be safe for older, non-pregnant patients, but could be toxic to children, pregnant women and their fetuses.
Kelsey had other worries about thalidomide as well. She wanted to know about the drug's mechanism of action – its effects on human metabolism, its chemistry and pharmacology, and its stability.[2] However, none of this data had been provided by Richardson-Merrell. There had been no chronic toxicity studies, excretion and absorption data was inadequate, and there were few manufacturing controls in place to assure quality.
Kelsey rejected the application and requested the aforementioned data from the company in a letter. Richardson-Merrell resubmitted the application but with no new information, and Kelsey turned it down again. She continued to request more data from the company, and with each request the 60 day clock was rewound to its beginning. As the end of 1960 approached, and with it the holiday season (the best time for selling sedatives)[3], Richardson-Merrell began ratcheting up the pressure on the FDA and Kelsey. Executives and scientists telephoned and personally visited Kelsey, and executives complained to her superiors that she was nit-picking and unreasonable.[1][5]
Still, Kelsey refused to clear Kevadon for sale in the United States until she could review satisfactory studies. She later said that the reports submitted by Grünenthal and Richardson-Merrell were more like testimonials than results of well-designed, controlled studies.[1] (One possible reason for the lack of data could be that Richardson-Merrell's "investigation" of thalidomide for its FDA application was organized and implemented not by scientists, but by the company's sales and marketing division.)[3] It wasn't enough to know how the drug acted in animals – she wanted to know how it worked in humans, and the data was not forthcoming. Kelsey had also heard anecdotal reports of peripheral neuropathy as a side effect of thalidomide, which only made her think more about the possible effects on a fetus. She continued to reject the Kevadon application. In total, the company resubmitted its Kevadon application to the FDA six times, but no new evidence was given in those applications and Kelsey refused to budge.
Tragedy
Unusual side effects had been reported by patients taking thalidomide in the UK, including peripheral neuropathy. Worse, pregnant women who had taken the drug were giving birth to babies with a condition called phocomelia – abnormally short limbs with toes sprouting from the hips and flipper-like arms. Other infants had eye and ear defects or malformed internal organs such as unsegmented small or large intestines. Chemie Grünenthal denied that thalidomide was responsible for any of these problems. In 1959, Grünenthal responded to one doctor's inquiry by saying, "We have no idea how these cases... could have been caused by Contergan."[3]
Under US law at the time, Richardson-Merrell had been legally distributing Kevdon on an "investigational" basis since early 1960, and pregnant women were included as patients after the first three months of the trial. American doctors were told,[3]
"We have firmly established the safety, dosage, and usefulness of Kevadon by both foreign and US laboratory and clinical studies. This program is designed to obtain widespread confirmation of its usefulness in a variety of hospitalized patients. Doctors need not report results if they don't want to..."
In December 1960, three months after Richardson-Merrell applied for FDA approval of Kevadon, the British Medical Journal published a letter from a British physician who reported cases of peripheral neuropathy, or painful extremities, in patients who had taken thalidomide over a long period of time. Kelsey read this letter, and she believed it was the first indication of toxicity effects.[5] It increased her misgivings about approving the application, and she immediately requested information from Richardson-Merrell about the problem. The company denied knowing about any harmful side effects. Years later, it was shown that Richardson-Merrell knew about the risk of nerve damage but failed to disclose the fact to the FDA.[3] Throughout 1961, more unofficial, anecdotal reports of thalidomide side effects surfaced in Europe and Australia.
On November 18, 1961, the German paper Welt am Sonntag published a letter by German pediatrician Widukind Lenz.[6] Lenz described more than 150 infants with malformations, including phocomelia, and associated them with thalidomide given to their mothers.[3] A stunning statistic was that 50 percent of the mothers with deformed children had taken thalidomide during the first trimester of pregnancy.[1] The limits of danger were amazingly narrow: women who took even one tablet of thalidomide between the 20th and 36th day after conception were at risk for delivering malformed infants – beyond that time, the drug caused no deformities at all.[3] Lenz notified Chemie Grünenthal about the dangers of its flagship product; ten days later, German authorities removed thalidomide from the market against Grünenthal's wishes. Grünenthal withdrew thalidomide soon afterward from the market and notified Richardson-Merrell of its decision.
In December, The Lancet published a letter by William McBride, an Australian physician, who noted large numbers of birth defects in the children of women who had taken thalidomide.[7] Other countries quickly pulled the drug from their stores and pharmacies. However, Grünenthal continued to dispute the claims that thalidomide was responsible for the defects, saying that their action was "merely a response to the sensationalism."[3]
Richardson-Merrill withdrew its Kevadon application in March 1962, but the sheer number and variety of brand names meant the drug remained available in some countries – thalidomide could be found in Brazil, Italy, and Japan as long as nine months after the German withdrawal.[8]
Unfortunately, Grünenthal's decision was too late for thousands of families. An estimated 8,000 to 12,000 infants were born with deformities caused by thalidomide, and of those only about 5,000 survived beyond childhood.[3] The medication never received approval for sale in the United States, but 2.5 million tablets had been given to more than 1,200 American doctors during Richardson-Merrell's "investigation," and nearly 20,000 patients received thalidomide tablets, including several hundred pregnant women. In the end, 17 American children were born with thalidomide-related deformities.[1] An estimated 40,000 people developed drug-induced peripheral neuropathy. Exact numbers will never be known because the companies and doctors kept incomplete and inaccurate records. Fortunately, no thalidomide victims have passed defects to their children, because thalidomide is not a mutagen.[9]
The ensuing American media coverage of thalidomide children, and the woman who refused to approve the drug for use in the United States, enveloped Kelsey and the FDA. Kelsey was praised in a story by Morton Mintz in The Washington Post on July 15, 1962. The headline read, " 'Heroine' of FDA Keeps Bad Drug Off of Market." Follow-up articles appeared in The New York Times, Life magazine, Saturday Review, and hundreds of other publications of the day.
More importantly, a controversial bill by U.S. Senator Estes Kefauver, of Tennessee, was resurrected and rewritten, passed by Congress, and signed by President John F. Kennedy on October 10, 1962. The Kefauver Harris Amendment strengthened the FDA's control of experimentation on humans and changed the way new drugs were approved and regulated. Before the thalidomide scandal, U.S. drug companies only had to show their new products were safe; for the first time, they would have to show their new drugs were safe and effective.[6] Informed consent was required of patients participating in clinical trials, and adverse drug reactions were required to be reported to the FDA.
The legal mess caused by the effects of thalidomide, and Chemie Grünenthal's shoddy testing and approval procedures, is still winding its way through courts worldwide even 50 years later. In connection with one of the first lawsuits, a court-ordered gag rule prohibited all mention of the thalidomide effects for ten years, from 1962 to 1972.[3] In one of the more recent developments in 2003, the Swedish government authorized an ex gratia compensation of SEK 250,000 for each of the 150 individuals that were subjected to thalidomide (marked in Sweden as Neurosedyn). According to a widely disputed investigation by the Swedish Office of the Chancellor of Justice (Justitiekanslern), no blame falls on the Swedish government. The Swedish government later decided to raise the compensation to SEK 500,000 each.
Thalidomide today
FDA Approval
"On May 26, 2006, the U.S. Food and Drug Administration granted accelerated approval for thalidomide (Thalomid, Celgene Corporation) in combination with dexamethasone for the treatment of newly diagnosed multiple myeloma (MM) patients." [1].htm Incredibly, the FDA approval came seven years after the first reports of efficacy in the medical literature (Desikan R, Munshi N, Zeldis J, et al. Activity of thalidomide (THAL) in multiple myeloma (MM) confirmed in 180 patients with advanced disease. Blood 1999;94:Suppl 1:603a-603a.abstract), and Celgene took advantage of "off-label" marketing opportunities to promote the drug in advance of its FDA approval for the myeloma indication. Thalomid, as the drug is commercially known, sold $300 million plus per year, while only approved for leprosy! [2] Thalidomide was and still is (2006) an important advance in the treatment of multiple myeloma, ever since news of its efficacy spread throughout the medical literature in 2000. The drug has some bothersome nuisance side effects such as neuropathy, constipation, and fatigue, but is likely more effective than chemotherapy for multiple myeloma. Thalidomide, along with another new drug, bortezomib, are changing the landscape of multiple myeloma treatment, such that toxic stem cell transplants may no longer be the standard treatment for this incurable malignancy.
Possible indications
Research on thalidomide slowed in the 1960s, but never completely stopped. The medication is now an example of how potentially dangerous chemical compounds can be used therapeutically with appropriate precautions and procedures.
In 1964, an Israeli physician named Jacob Sheskin was trying to help a critically-ill French patient with erythema nodosum leprosum (ENL), a very painful complication of leprosy. He looked throughout his small hospital for anything that might help his patient stop aching long enough to sleep. He came across a bottle of thalidomide tablets, and remembered that the drug had been effective in helping mentally ill patients sleep – and also that it was banned. Thinking he had nothing to lose, Sheskin gave the man two tablets of thalidomide. The patient slept for hours, and he felt good enough to get out of bed without aid when he woke up. The result was soon followed by more favorable experiences, followed by a clinical trial. Dr. Sheskin's drug of last resort revolutionized the care of leprosy, and led to the closing of most leprosy hospitals.[3]
Serious infections including sepsis and tuberculosis cause the level of tumor necrosis factor α (TNFα) to rise. TNFα is a chemical mediator in the body, and it may enhance the wasting process in cancer patients as well. Thalidomide may reduce the levels of TNFα, and it is possible that the drug's effect on ENL is caused by this mechanism.[2]
Thalidomide also has potent anti-inflammatory effects that may help ENL patients. In July 1998, the FDA approved the application of Celgene to distribute thalidomide under the brand name Thalomid for treatment of ENL. Celgene received approval for its use against multiple myeloma in Australia in 2003 and in the US in 2005. [6] Thalomid, in conjunction with dexamethasone, is now standard therapy for multiple myeloma.
Thalidomide also inhibits the growth of new blood vessels (angiogenesis), which may be useful in treating macular degeneration and other diseases; this effect also helps AIDS patients with Kaposi's sarcoma, although there are better and cheaper drugs to treat the condition. Also for AIDS patients, thalidomide may be able to fight painful, debilitating aphthous lesions in the mouth and esophagus which prevent sufferers from eating. The FDA formed a Thalidomide Working Group in 1994 to provide consistency between its divisions, with particular emphasis on safety monitoring. The agency also imposed severe restrictions on the distribution of Thalomid through the System for Thalidomide Education and Prescribing Safety (STEPS) program.[2]
Thalidomide is also being investigated for treating symptoms of prostate cancer, glioblastoma, lymphoma, arachnoiditis, Behçet's disease, and Crohn's disease. In a small trial, Australian researchers found thalidomide sparked a doubling of the number of T cells in patients, allowing the patients' own immune system to attack cancer cells.
Teratogenic mechanism
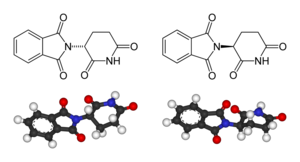
Left: (R)-thalidomide
Right: (S)-thalidomide
Thalidomide is racemic – it contains both left- and right-handed isomers in equal amounts. One enantiomer is effective against morning sickness. The other is teratogenic, and causes birth defects. The enantiomers are converted to each other in vivo – that is, if a human is given (R)-thalidomide or (S)-thalidomide, both isomers can be found in the serum – therefore, administering only one enantiomer will not prevent the teratogenic effect in humans.
The mechanism by which thalidomide causes birth defects is not completely understood, although recent papers suggest that it alters TNFα production, modulates integrins, alters T-cell ratios and inhibits angiogenesis. It is known to intercalate into DNA at guanine sites, which may alter expression of certain genes thereby causing the dramatic malformations.
Side effects
Apart from its infamous tendency to induce birth defects and peripheral neuropathy, the main side effects of thalidomide include fatigue and constipation. It also is associated with an increased risk of deep vein thrombosis especially when combined with dexamethasone, as it is for treatment of multiple myeloma. High doses can lead to pulmonary edema, atelectasis, aspiration pneumonia, and refractory hypotension.
Thalidomide analogs
The exploration of the antiangiogenetic and immunomodulatory activities of thalidomide has led to the study and creation of thalidomide analogs. In 2005, Celgene received FDA approval for lenalidomide (Revlimid) as the first commercially useful derivative. Revlimid is only available in a restricted distribution setting to avoid its use during pregnancy. Further studies are conducted to find safer compounds with useful qualities. Another analog, Actimid, is in the clinical trial phase. These thalidomide analogs can be used to treat different diseases, or used in a regimen to fight two conditions.
Notable children of thalidomide
- Theresia Degener, a prominent human rights lawyer
with a special interest in the rights of the disabled
Mat Fraser, a comedian, actor, co-presenter of the BBC's Ouch Podcast
Alvin Law, a motivational speaker and former radio broadcaster.
Tony Melendez is a guitarist who was born without arms. He plays only with his feet.
Brett Nielsen , a musician, was the first Australian thalidomide child
Thomas Quasthoff is an internationally acclaimed bass-baritone who describes himself : "1.34 meters tall, short arms, seven fingers - four right, three left - large, relatively well formed head, brown eyes, distinctive lips; profession: singer."
Thalidomide in literature, music & arts
- On Giant's Shoulders: The Story of Terry Wiles (ISBN
0-7230-0146-4) by Marjorie Wallace, a book & movie about
the life of Terry Wiles
Peter Milligan and Brendan McCarthy's short graphic novel Skin (1992) features a teenage skinhead who is a child of thalidomide.
The horror movie Scanners deals with children whose mothers took a similar "wonder drug" during their pregnancy with even more dramatic side effects.
The Punk rock band NOFX wrote a song entitled "Thalidomide Child".
The song "We Didn't Start the Fire" by Billy Joel mentions thalidomide.
The novel Mount Dragon by Douglas Preston and Lincoln Child contains a computer hacker character named Mime who is a thalidomide survivor.
The poem Thalidomide by Sylvia Plath
The novel All Families are Psychotic' by Douglas Coupland refers to thalidomide.
The short story Fortune's Always Hiding by Irvine Welsh in the novel Ecstasy: Three Tales of Chemical Romance (1996) is a story of revenge-seeking thalidomide victims.
References
- ^ a b c d e f Bren, Linda. "Frances Oldham Kelsey: FDA Medical Reviewer Leaves Her Mark on History", FDA Consumer, US Food and Drug Administration, 2001-02-28. Retrieved on 2006-09-21.
- ^ a b c d Burkholz, Herbert. "Giving Thalidomide a Second Chance", FDA Consumer, US Food and Drug Administration, 1997-09-01. Retrieved on 2006-09-21.
- ^ a b c d e f g h i j k l m n o p q Silverman, MD, William (2002-04-22). "The Schizophrenic Career of a "Monster Drug"". Pediatrics 110 (2): 404-406. Retrieved on 2006-09-21.
- ^ Sjostrom, Henning, Nilsson, Robert (1972). Thalidomide and the power of the drug companies. Hammondsworth: Penguin. ISBN 0140522980.
- ^ a b c d Karen Geraghty (July 2006). Profile of a Role Model. AMA (Virtual Mentor). American Medical Association. Retrieved on 2006-09-21.
- ^ a b c d Rouhi, Maureen. Thalidomide. Chemical & Engineering News. American Chemical Society. Retrieved on 2006-09-21.
- ^ Thalidomide and congenital abnormalities. The Lancet. James Lind Library (December 1961). Retrieved on 2006-09-21.
- ^ Lenz, Widukind. The History of Thalidomide. 1992 UNITH Congress address. Thalidomide Victims Association of Canada. Retrieved on 2006-09-21.
- ^ Smithells, Dick (Nov 1998). "Does Thalidomide Cause Second Generation Birth Defects?". Drug Safety 19 (5): 339-341. Retrieved on 2006-09-21.
Further reading
- Stephens, Trent, Brynner, Rock (2001-12-24). Dark Remedy: The Impact of Thalidomide and Its Revival as a Vital Medicine. Perseus. ISBN 0-7382-0590-7.
- Knightley, Phillip, Evans, Harold. Potter, Elaine. Wallace, Marjorie. (1979). Suffer The Children: The Story of Thalidomide. New York: The Viking Press. ISBN 0-670-68114-8.
External links
- Thalidomide product monograph
- Multiple Myeloma Research Foundation article on Thalidomide
- International Myeloma Foundation article on Thalidomide
- Thalidomide — Annotated List of Links (covering English and German pages)
- WHO Pharmaceuticals Newsletter No. 2, 2003 - See page 11, Feature Article
- Celgene website on Thalomid
- The Return of Thalidomide - BBC
- CBC Digital Archives – Thalidomide: Bitter Pills, Broken Promises




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xxx
216.73.xxx.xxx
 Server Time:
Server Time: