 |
|
|
Tetracycline
|
|
| Systematic (IUPAC) name | |
|
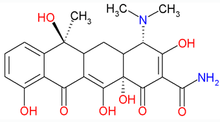
2-(amino-hydroxy-methylidene)-4-dimethylamino- 6,10,11,12a-tetrahydroxy-6-methyl-4,4a,5, 5a-tetrahydrotetracene-1,3,12-trione OR 4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro- 3,6,10,12,12a-pentahydroxy- 1,11dioxo-naphthacene-2carboxamide |
|
| Identifiers | |
| CAS number | 60-54-8 |
| ATC code | A01AB13 D06AA04 J01AA07 S01AA09 S02AA08 S03AA02 |
| PubChem | 643969 |
| DrugBank | APRD00572 |
| Chemical data | |
| Formula | C22H24N2O8 |
| Mol. weight | 444.435 g/mol |
| Pharmacokinetic data | |
| Bioavailability | 60-80% Oral, while fasting <40% Intramuscular |
| Metabolism | Not metabolised |
| Half life | 6-11 hours |
| Excretion | Fecal and Renal |
| Therapeutic considerations | |
| Pregnancy cat. | D(AU) D(US) |
| Legal status | ℞ Prescription only |
| Routes | oral, topical (skin & eye), im, iv |
Tetracycline (INN) (IPA: [ ˌtɛtrəˈsaɪklin ]) is a broad-spectrum antibiotic produced by the streptomyces bacterium, indicated for use against many bacterial infections. It is commonly used to treat acne. It is sold under the brand names SumycinŽ;, TetracynŽ; and PanmycinŽ, among others. ActisiteŽ is a thread-like fiber form, used in dental applications. It is also used to produce several semi-synthetic derivatives, which together are known as the Tetracycline antibiotic group. It works by inhibiting action of the prokaryotic 30S ribosome. Toxicity may be result of inactivation of mitochondrial 30S ribosomes in host cells.
Contents |
History
Tetracycline was first discovered by Lloyd Conover in the research departments of Pfizer. The patent for Tetracycline was first issued in 1950 (patent number 2,624,354). Tetracycline sparked the development of many chemically altered antibiotics and in doing so has proved to be one of the most important discoveries made in the field of antibiotics.
Cautions, Contraindications, Side effects
Are as those of the Tetracycline antibiotics group:
- Can stain developing teeth (even when taken by the
mother during pregnancy)
Inactivated by Ca2+ ion, not advised to be taken with milk or yogurt
Skin photosensitivity, not advised to be exposed to the Sun or intense light
Drug induced lupus, and hepatitis
Tinnitus
Indication
Tetracycline's primary use is for the treatment of acne vulgaris and rosacea.
It is also used to treat a very wide range of infections; see Tetracycline antibiotics for details.
Other uses
Since tetracycline is absorbed into bone, it is used as a marker of bone growth for biopsies in humans, and as a biomarker in wildlife to detect consumption of medicine- or vaccine-containing baits. The presence of tetracyline in bone is detected by its fluorescence.
References
- Mayton CA. Tetracycline labeling of bone
- Olson CA, et al. Bait ingestion by free-ranging raccoons and nontarget species in an oral rabies vaccine field trial in Florida. J Wildl Dis. 2000 Oct;36(4):734-43.




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xxx
216.73.xxx.xxx
 Server Time:
Server Time: