| Sorbitol | |
|---|---|
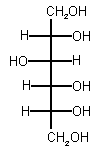 |
|
| Chemical name | Sorbitol |
| E number | E 420 |
| Chemical formula | C6H14O6 |
| Molecular mass | 182.17 g/mol |
| Melting point | 95 °C |
| Boiling point | 296 °C |
| Density | 0.68 g/cm³ |
| CAS number | [50-70-4] |
| SMILES | OCC(O)C(O)C(O)C(O)CO |
| IUPAC name | hexane-1,2,3,4,5,6-hexaol |
Sorbitol, also known as glucitol, is a sugar alcohol the body metabolises slowly. It is obtained by reduction of glucose changing the aldehyde group to an additional hydroxyl group hence the name sugar alcohol.
Sorbitol is used in various cough syrups, and is usually listed under the inactive ingredients. There is a growing opinion within the medical community that it should be listed as an active ingredient, because too much Sorbitol (about 50g or more for adults) can cause severe gastro-intestinal problems.
Sorbitol is a sugar substitute often used in diet foods (including diet drinks) and sugar-free chewing gum. It also occurs naturally in many stone fruits. Sorbitol is also referred to as a nutritive sweetener because it provides calories or energy to the diet - 2.6 calories (11 kilojoules) per gram versus the average 4 calories (17 kJ) of sugar and starch, while retaining 60% of the sweetness.
Sorbitol is produced naturally by the body, yet sorbitol is poorly digested by the body. Too much sorbitol in cells can cause damage.
Diabetic retinopathy and neuropathy may be related to excess sorbitol in the cells of the eyes and nerves. The source of this sorbitol in diabetics is excess glucose, which goes through the polyol pathway. Ingesting large amounts of sorbitol can lead to some abdominal pain, gas, and mild to severe diarrhea. Sorbitol can also aggravate irritable bowel syndrome and fructose malabsorption.
Sorbitol is often used in modern cosmetics as a humectant and thickener. Some transparent gels can only be made with sorbitol as it has a refractive index sufficiently high for transparent formulations. It is also used as a humectant in some cigarettes.
Sorbitol is used as a cryoprotectant additive (mixed with sucrose and sodium polyphosphates) in the manufacture of surimi, a highly refined, uncooked fish paste most commonly produced from Alaska (or walleye) pollock (Theragra chalcogramma).
Sorbitol is identified as a potential key chemical intermediate [1] from biomass resources. Complete reduction of sorbitol opens the way to alkanes such as hexane which can be used as a biofuel. Sorbitol itself provides much of the hydrogen required for the transformation.
- 19 C6O6H14 → 13 C6H14 + 36 CO2 + 42 H2O
The above chemical reaction is exothermic and 1.5 mole of sorbitol generates 1 mole of hexane. When hydrogen is co-fed no carbon dioxide production takes place.
External links
- NIH Diabetes dictionary — see entry on sorbitol




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xxx
216.73.xxx.xxx
 Server Time:
Server Time: