 |
|
|
Simvastatin
|
|
| Systematic (IUPAC) name | |
|
[(1S,3R,7R,8S,8aR)-8-[2-[(2R,4R)-4-hydroxy-6- oxo-oxan-2-yl]ethyl]-3,7-dimethyl-1,2,3,7,8,8a -hexahydronaphthalen-1-yl]2,2-dimethylbutanoate |
|
| Identifiers | |
| CAS number | 79902-63-9 |
| ATC code | C10AA01 |
| PubChem | 54454 |
| DrugBank | APRD00104 |
| Chemical data | |
| Formula | C25H38O5 |
| Mol. weight | 418.566 g/mol |
| Pharmacokinetic data | |
| Bioavailability | 5% |
| Protein binding | 95% |
| Metabolism | Hepatic (CYP3A4) |
| Half life | 3 hours |
| Excretion | Renal 13%, faecal 60% |
| Therapeutic considerations | |
| Pregnancy cat. | D(AU) X(US) |
| Legal status | S4(AU) P(UK) ℞-only(US) |
| Routes | Oral |
Simvastatin (INN) (IPA: [ˈsɪmvəˌstætən]) is a hypolipidemic drug belonging to the class of pharmaceuticals called "statins". It is used to control hypercholesterolemia (elevated cholesterol levels) and to prevent cardiovascular disease. Simvastatin is a synthetic derivate of a fermentation product of Aspergillus terreus.
Contents |
History
The development of simvastatin was closely linked with the research and development of lovastatin. Biochemist Jesse Huff and his colleagues at Merck began researching the biosynthesis of cholesterol in the early 1950s. In 1956, mevalonic acid was isolated from a yeast extract by Karl Folkers, Carl Hoffman, and others at Merck; while Huff and his associates confirmed that mevalonic acid was an intermediate in cholesterol biosynthesis. In 1959, the HMG-CoA reductase enzyme (a major contributor of internal cholesterol production) was discovered by researchers at the Max Planck Institute. This discovery encouraged scientists worldwide to find an effective inhibitor of this enzyme.
By 1976, Akira Endo had isolated the first inhibitor (compactin ML-236B) from the fungus, Penicillium citrinium in Sankyo, Japan[1]. In 1979, Hoffman and colleagues isolated lovastatin from a strain of the fungus Aspergillus terreus. While developing and researching lovastatin, Merck scientists synthetically derived a more potent HMG-CoA reductase inhibitor from a fermentation product of Aspergillus terreus, which was designated MK-733 (later to be named simvastatin).[2]
Uses
Simvastatin is a powerful lipid-lowering drug that can decrease low density lipoprotein (LDL) levels by up to 50%. It is used in doses of 5 mg up to 80 mg. Higher doses (160 mg) have been found to be too toxic, while giving only minimal benefit in terms of lipid lowering. There is no real effect on HDL and triglyceride levels.
From recent research it has become apparent that simvastatin and other statins inhibit the progression of atherosclerosis beyond their effects on LDL. A large number of explanations has been proposed, for example its inhibitory effect on macrophages in the atherosclerotic plaque lesions.
Rationing
Since its introduction, there has been a large debate surrounding the price for lipid-lowering treatment and its benefits on atherosclerosis. Although this has affected the other statins as well, simvastatin was the first statin drug to be used extensively in clinical practice.
A number of large epidemiological studies were conducted to discover which patients would benefit most from statin drugs; most studies involve simvastatin as the study drug. The most influential studies were the Scandinavian Simvastatin Survival Study (4S) and the Heart protection study (HPS).
It has now become apparent that patients with one or more risk factors for cardiovascular disease (such as diabetes mellitus, hypertension or a positive family history) can benefit from statins—even if they do not have substantially elevated cholesterol levels.
Simvastatin was introduced in the late 1980s, and in many countries it is now available as a generic preparation. This has led to a decrease of the price of most statin drugs, and a reappraisal of the health economics of preventive statin treatment.
In the UK, simvastatin (in a dose of 10mg) has recently become available to purchase from pharmacies without prescription.
Pharmacology
All statins act by inhibiting HMG-CoA reductase, the rate-limiting enzyme of the HMG-CoA reductase pathway, the metabolic pathway responsible for the endogenous production of cholesterol.
The drug is the form of an inactive lactone that is hydrolized after ingestion to produce the active agent. It is a white, nonhygroscopic, crystalline powder that is practically insoluble in water, and freely soluble in chloroform, methanol and ethanol.
Interactions
Grapefruit contains the flavanones naringenin and bergamottin, which inhibit the liver cytochrome P450 3A4. This in turn slows metabolization of simvastatin and a large number of other drugs. Therefore, patients taking simvastatin should restrict their intake of grapefruit and grapefruit-containing products. [3]
Marketing
Reference: Drug Discovery Today editorial, 2005.[4]
Brand names: Zocor®, Zocor Heart Pro®, marketed by the pharmaceutical company Merck & Co. and Denan (Germany), Liponorm, Sinvacor, Sivastin (Italy), Lipovas (Japan), Lodales (France), Zocord (Austria and Sweden), Zimstat, Simvahexal and Lipex (Australia) and other.
The primary US patent for Zocor expired on June 23, 2006; Ranbaxy Laboratories (at the 80-mg strength) and Teva Pharmaceutical Industries thru its Ivax Pharmaceuticals unit (at all other strengths) were given approval by the FDA to manufacture and sell simvastatin as a generic drug with 180-day exclusivity. Dr. Reddy's Laboratories also has a license from Merck & Co. to sell simvastatin as an authorized generic drug.
Ezetimibe/simvastatin is a combination product to lower lipids and marketed as Vytorin.
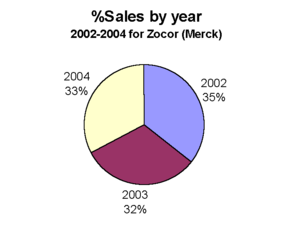
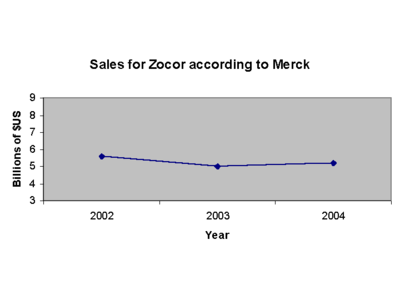
Sales
Prior to losing U.S. patent protection, simvastatin was Merck & Co.’s largest selling drug and second largest selling cholesterol lowering drug in the world; it recorded 4.3 billion dollars of sales in 2005.[4] Zocor had an original patent expiration date of January 2006 but was extended by the United States Federal Drug Administration (FDA) o expire on June 23, 2006. The FDA granted the patent extension after Merck & Co, Inc. submitted data from studies of the drug’s positive effect on children, a move typically used by drug companies to lengthen exclusivity.[4]
Ordinarily, Merck & Co. would have expected a sharp decrease in sales after the generic versions of simvastatin entered the market; however, Merck has slashed the price of Zocor dramatically in an effort to claim sales that would have otherwise gone to the generic versions. At least two major U.S. health insurers, UnitedHealthcare and WellPoint, are now offering Zocor to their members at generic copays.[5]
In addition, since Merck & Co. itself manufactures at least some versions of Dr. Reddy's authorized generic simvastatin, and since only Dr. Reddy's can supply generic simvastatin in all available strengths (unlike Teva and Ranbaxy), Merck & Co. is also poised to profit from the Dr. Reddy's version. An 80mg, 30-count bottle of Dr. Reddy's simvastatin obtained July 6, 2006, states it is made by Merck Sharp & Dohme (Merck & Co.'s name outside the US to avoid conflicts with Merck KGaA) in the UK, just like 80mg Zocor, and has a Merck & Co. logo on the bottom; except for omitting the "80" on one side, the pills are visually identical to 80mg Zocor, including "543" on the other side which is the key part of the National Drug Code for 80mg Zocor.
References
- ^ Liao and Laufs. Pleiotropic Effects of Statins.(2005) Annu. Rev. Pharmacol.Toxicol:45:89-118
- ^ Olivia Williams, Anne-Marie Jacks, Jim Davis, Sabrina Martinez (1998). “Case 10: Merck(A): Mevacor*”, Ed. Allan Afuah Innovation Management - Strategies, Implementation, and Profits. Oxford University Press. Retrieved on 2006-07-19.
- ^ Grapefruit (English). Retrieved on 2006-09-15.
- ^ a b c Maggon, Krishan. "Best-selling human medicines 2002-2004 (editorial)". 2005. Drug Discovery Today, 10(11):739-742
- ^ Brin, Dinah Wisenberg. "Zocor Patent Expiring Means Bidding War", Associated Press, 2006-06-22. Retrieved on 2006-07-09.
- http://research.businessweek.com/business_summary.asp?Symbol=mrk
- http://money.cnn.com/2002/02/27/companies/merck/#TOP
External links
- RxList.com - Simvastatin




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xxx
216.73.xxx.xxx
 Server Time:
Server Time: