 |
|
 |
|
|
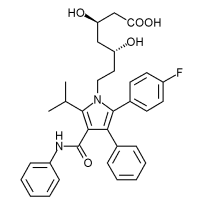
Atorvastatin
|
|
| Systematic (IUPAC) name | |
| [R-(R*, R*)]-2-(4-fluorophenyl)-beta, delta-dihydroxy-5- (1-methylethyl)-3-phenyl-4- [(phenylamino)carbonyl]-1H- pyrrole-1-heptanoic acid | |
| Identifiers | |
| CAS number | 134523-00-5 |
| ATC code | C10AA05 |
| PubChem | 60823 |
| DrugBank | APRD00055 |
| Chemical data | |
| Formula | C33H34FN2O5 |
| Mol. weight | 558.64 |
| Pharmacokinetic data | |
| Bioavailability | 12% |
| Metabolism | Liver |
| Half life | 14 hours |
| Excretion | Bile |
| Therapeutic considerations | |
| Pregnancy cat. | D(AU) X(US) |
| Legal status | S4(AU) POM(UK) ℞-only(US) |
| Routes | oral |
Atorvastatin (INN) (IPA: [əˈtɔvəˌstætn]) is a member of the drug class known as statins, used for lowering cholesterol and thereby reducing cardiovascular disease. Atorvastatin inhibits a rate-determining enzyme located in hepatic tissue used in cholesterol synthesis, which lowers the amount of cholesterol produced. This also has the effect of lowering the total amount of LDL cholesterol.
With 2005 sales of US$12.2 billion under the brand name Lipitor, it is the largest selling drug in the world.
Contents |
Pharmacology
As with other statins, atorvastatin is a competitive inhibitor of HMG-CoA reductase. Unlike most others, however, it is a completely synthetic compound. HMG-CoA reductase catalyzes the reduction of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) to mevalonate, which is the rate-limiting step in hepatic cholesterol biosynthesis. Inhibition of the enzyme decreases de novo cholesterol synthesis, increasing expression of low-density lipoprotein receptors (LDL receptors) on hepatocytes. This increases the LDL uptake by the hepatocytes, decreasing the amount of LDL in the blood.
Clinical use
Indications
Atorvastatin is indicated as an adjunct to diet for the treatment of dyslipidaemia, specifically hypercholesterolaemia. It has also been used in the treatment of combined hyperlipidemia.[1]
Available forms
Atorvastatin calcium tablets are currently marketed by Pfizer under the trade name Lipitor, in tablets (10, 20, 40 or 80 mg) for oral administration. Tablets are white, elliptical, and film coated. In some countries it may also be known as: Sortis, Torvast, Totalip, Tulip, Xarator or Liprimar.
Adverse effects
-
For additional information see: Statin#Safety
Common adverse drug reactions (≥1% of patients) associated with atorvastatin therapy include: myalgia, mild transient gastrointestinal symptoms, elevated hepatic transaminase concentrations, headache, insomnia, and/or dizziness.[1]
Myopathy and rhabdomyolysis occur in <0.1% of patients. Risk is increased in patients with renal impairment, serious concurrent illness; and/or concomitant use of drugs which inhibit CYP3A4.[1]
References
External links
- Lipitor.com – manufacturer's site
- MedlinePlus Drug information: Atorvastatin (Systemic) – information from USP DI Advice for the Patient
{{ChemicalSources
Further reading
- Maggon, Krishan. "Best-selling human medicines 2002-2004 (editorial)". 2005. Drug Discovery Today, 10(11):739-742. DOI:10.1016/S1359-6446(05)03468-9
- Lipitor: Prescribing Information. (2004) Pfizer Ireland Pharmaceuticals.




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xxx
216.73.xxx.xxx
 Server Time:
Server Time: