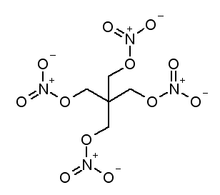
 PETN |
|
| 1,3-Dinitrato-2,2-bis (nitratomethyl)propane IUPAC name |
|
| Chemical formula | C5H8N4O12 |
| Molecular mass | 316.14 g/mol |
| Shock sensitivity | Medium |
| Friction sensitivity | Medium |
| Density | 1.773 kg/m³ at 20 °C |
| Explosive velocity | 8,400 m/s |
| RE factor | 1.66 |
| Melting point | 141.3 °C |
| Autoignition temperature | Decomposes at 190 °C |
| Appearance | Odourless white crystalline solid. |
| CAS number | 78-11-5 |
| PubChem | {{{PubChem}}} |
PETN (Pentaerythritol Tetranitrate, also known as Penthrite) is one of the strongest known high explosives, with a relative effectiveness factor (R.E. factor) of 1.66. It is more sensitive to shock or friction than TNT or tetryl, and it is never used alone as a booster. It is primarily used in booster and bursting charges of small caliber ammunition, in upper charges of detonators in some land mines and shells, and as the explosive core of detonation cord.
PETN is one of the explosive ingredients used in Semtex plastic explosive. During World War II the M9A1 2.36" Rocket Launcher (Bazooka) shaped charge, with 8 oz of pentolite (a mixture of PETN and TNT), could penetrate up to 5 inches of armor.
Demolition charge, M118, commonly called Flex-X or sheet explosive, consists of 4 half-pound sheets of flexible explosive packed in a plastic envelope. Each sheet is approximately 3 inches wide, 12 inches long, and ¼ inch thick. Note: The exact explosive contained in an M118 charge varies with the manufacturer. At present, some manufacturers use PETN as the basic explosive. Others use RDX. Charges manufactured in the future may include other explosives.
PETN is also used as a vasodilator. The medicine for heart diseases, "Lentonitrat", is pure PETN.
PETN was also used by Richard Reid for the plot to destroy American Airlines Flight 63 by trying to ignite explosives hidden in his shoes.
Contents |
Properties
The velocity of detonation of PETN at a density of 1.7 is 8,400 meters per second.
PETN's formula is C(CH2ONO2)4. Its theoretical maximum crystal density is 1.773 g/cm3. It melts toward 141 °C.
As a pollutant in the environment
PETN does not occur naturally, so the production and use of this kind of compound can lead to contamination of the environment. PETN is subject to biodegradation in untreated or unpreserved urine and feces. There also have been some reports of its degradation by bacteria, whose PETN reductase denitrates PETN into trinitrates and then dinitrates (French et al., 1996). The last compound shown in the pathway, pentaerythritol dinitrate, is degraded further to unknown products.
Production
PETNs' preparation involves the nitration of
pentaerythritol with a mixture of concentrated nitric and
sulfuric acid. The preferred method of nitration is the ICI method, which utilizes concentrated nitric acid (98%+)
alone, as mixed acid can create unstable sulfonated
by-products.
C(CH2OH)4 + 4HNO3 → C(CH2ONO2)4 + 4H2O
References
Cooper, Paul W., Explosives Engineering, New York: Wiley-VCH, 1996. ISBN 0-471-18636-8




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 14.227.xxx.xxx
14.227.xxx.xxx
 Server Time:
Server Time: