 |
|
 |
|
|
Heparin
|
|
| Identifiers | |
| CAS number | 9005-49-6 |
| ATC code | B01AB01 C05BA03 S01XA14 |
| PubChem | 772 |
| DrugBank | APRD00056 |
| Chemical data | |
| Formula | mixed polymeric (for skeletal structure shown above C12H19NO20S3) |
| Mol. weight | 12000-15000 g/mol |
| Pharmacokinetic data | |
| Bioavailability | nil |
| Metabolism | hepatic |
| Therapeutic considerations | |
| Routes | i.v., s.c. |
Heparin is a highly sulfated glycosaminoglycan widely used as an injectable anticoagulant. It is also used to form an inner anticoagulant surface on various experimental and medical devices such as test tubes and renal dialysis machines. Pharmaceutical grade heparin is commonly derived from mucosal tissues of slaughtered meat animals such as porcine intestine or bovine lung.[1]
Contents |
Heparin structure
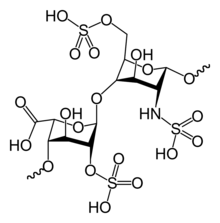
Native heparin is a polymer with a molecular weight ranging from 3 kDa to 40 kDa although the average molecular weight of most commercial heparin preparations is in the range of 12 kDa to 15 kDa. Heparin is a member of the glycosaminoglycan family of carbohydrates (which includes the closely related molecule heparan sulfate) and consists of a variably sulfated repeating disaccharide unit. The main disaccharide units that occur in heparin are shown below. The most common disaccharide unit is composed of a 2-O-sulfated iduronic acid and 6-O-sulfated, N-sulfated glucosamine, IdoA(2S)-GlcNS(6S). For example this makes up 85% of heparins from beef lung and about 75% of those from porcine intestinal mucosa. Not shown below are the rare disaccaharides containing a 3-O-sulfated glucosamine (GlcNS(3S,6S) or a free amine group (GlcNH3+). Under physiological conditions the ester and amide sulfate groups are deprotonated and attract positively charged counterions to form a heparin salt. It is in this form that heparin is usually administered as an anticoagulant.
Abbreviations
- GlcA = β-L-glucuronic acid
- IdoA = α-L-iduronic acid
- IdoA(2S) = 2-O-sulfo-α-L-iduronic acid
- GlcNAc = 2-deoxy-2-acetamido-α-D-glucopyranosyl
- GlcNS = 2-deoxy-2-sulfamido-α-D-glucopyranosyl
- GlcNS(6S) = 2-deoxy-2-sulfamido-α-D-glucopyranosyl-6-O-sulfate
Three-dimensional structure
The three dimensional structure of heparin is complicated by the fact that iduronic acid may be present in either of two low energy conformations when internally positioned within an oligosaccharide. The conformational equilibrium being influenced by sulfation state of adjacent glucosamine sugars.[2] Nevertheless the solution structure of a heparin dodecasacchride composed solely of six GlcNS(6S)-IdoA(2S) repeat units has been determined using a combination of NMR spectroscopy and molecular modelling techniques.[3] Two models were constructed one in which all IdoA(2S) were in the 2S0 conformation (A and B below) and one in which they are in the 1C4 conformation (C and D below). However there is no evidence to suggest changes between these conformations occurs in a concerted fashion. These models correspond to the protein data bank code 1HPN
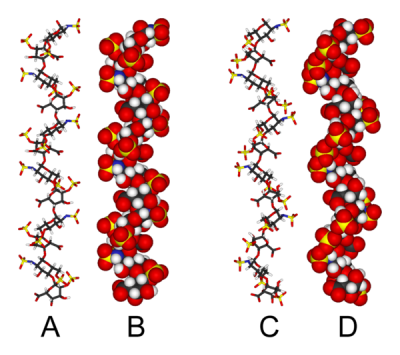
In the image above:
- A = 1HPN (all IdoA(2S) residues in 2S0 conformation) Jmol viewer
- B = van der Waals radius space filling model of A
- C = 1HPN (all IdoA(2S) residues in 1C4 conformation) Jmol viewer
- D = van der Waals radius space filling model of C
In these models heparin adopts a helical conformation, the rotation of which places clusters of sulfate groups at regular intervals of about 17 angstroms (1.7 nm) on either side of the helical axis.
Medical use
Heparin acts as an anticoagulant, preventing the formation of clots and extension of existing clots within the blood. While heparin does not break down clots that have already formed, it allows the body's natural clot lysis mechanisms to work normally to break down clots that have already formed. Heparin is used for anticoagulation for the following conditions:
- Acute coronary syndrome, e.g., myocardial infarction
Atrial fibrillation
Deep-vein thrombosis and pulmonary embolism.
History
Heparin is one of the oldest drugs currently still in widespread clinical use. As it was discovered in 1916, its introduction predates the establishment of the United States Food and Drug Administration.[4] It was originally isolated from liver cells, hence its name (hepar or "ηπαρ" is Greek for "liver"). Scientists were looking for an anticoagulant that could work safely in humans, and Jay McLean, a second-year medical student from Johns Hopkins University working under the guidance of William Henry Howell, found a compound extracted from liver that acted as an anticoagulant.
Administration
Heparin is given parenterally; it is digested when taken by mouth. It can be injected intravenously or subcutaneously (under the skin). Intramuscular injections (into muscle) are avoided because of the potential for forming hematomas. Because of its short biologic half-life of approximately one hour, heparin must be given frequently or as a continuous infusion.
If long-term anticoagulation is required, heparin is often only used to commence anticoagulation therapy until the oral anticoagulant warfarin takes effect.
Adverse reactions
A serious side-effect of heparin is heparin-induced thrombocytopenia (HIT syndrome). HITS is caused by an immunological reaction that makes platelets aggregate within the blood vessels, thereby using up coagulation factors. Formation of platelet clots can lead to thrombosis, while the loss of coagulation factors and platelets may result in bleeding. HITS can (rarely) occur shortly after heparin is given, but also when a person has been on heparin for a long while. Immunologic tests are available for the diagnosis of HITS. There is also a benign form of thrombocytopenia associated with early heparin use which resolves without stopping heparin.
Other side effects include alopecia and osteoporosis.
As with many drugs, overdoses of Heparin can be fatal. In September 2006, Heparin received worldwide publicity when 3 prematurely-born infants died after they were mistakenly given adult-sized doses of Heparin at an Indianapolis hospital.[5]
Treatment of overdose
In case of overdose, protamine sulfate can be given to counteract the action of heparin.
Mechanism of action
Heparin is a naturally occurring anticoagulant produced by basophils and mast cells. [6] Heparin binds to the enzyme inhibitor antithrombin III (AT-III) causing a conformational change which results in its active site being exposed. The activated AT-III then inactivates thrombin and other proteases involved in blood clotting, most notably factor Xa. The rate of inactivation of these proteases by AT-III increases 1000-fold due to the binding of heparin.[7]
AT-III binds to a specific pentasaccharide sulfation sequence contained within the heparin polymer
GlcNAc/NS(6S)-GlcA-GlcNS(3S,6S)-IdoA(2S)-GlcNS(6S)
The conformational change in AT-III on heparin binding mediates its inhibition of factor Xa. For thrombin inhibition however, thrombin must also bind to the heparin polymer at a site proximal to the pentasacchride. The formation of a ternary complex between AT-III, thrombin and heparin results in the inactivation of thrombin. For this reason heparin's activity against thrombin is size dependent, the ternary complex requiring at least 18 sacchride units for efficient formation.[8] In contrast anti factor Xa activity only requires the pentasacchride binding site.
This size difference has led to the development of low molecular weight heparins and more recently to fondaparinux (a synthetic pentasacchride identical in structure to the AT-III binding pentasacchride) as pharmaceutical anticoagulants. These products target anti factor Xa activity rather than anti thrombin (IIa) activity with the aim of facilitating a more subtle regulation of coagulation and an improved therapeutic index.
With these fractioned heparin alternatives, there is a reduced risk of osteoporosis and heparin-induced thrombocytopenia (HIT). Monitoring of the APTT is also not required and indeed does not reflect the anticoagulant effect, as APTT is insensitive to alterations in factor Xa.
Danaparoid, a mixture of heparan sulfate, dermatan sulfate and chondroitin sulfate can be used as an anticoagulant in patients who have developed HIT. Because danaparoid does not contain heparin or heparin fragments cross-reactivity of danaparoid with heparin-induced antibodies is reported as less than 10% (see here)
The effects of heparin are measured in the lab by the partial thromboplastin time (aPTT), (the time it takes the blood plasma to clot).
Heparin's exact physiological role is still unclear, because blood anti-coagulation is mostly achieved by endothelial cell-derived heparan sulfate proteoglycans.[9]
Heparin gel
Heparin gel (topical) may sometimes be used to treat sports injuries. It is known that the diprotonated form of histamine binds site specifically to heparin.[10] The release of histamine from mast cells at a site of tissue injury contributes to an inflammatory response. The rationale behind the use of such topical gels may be to block the activity of released histamine and so help to reduce inflammation.
Other uses/information
- Test tubes, Vacutainers, and capillary tubes that use the lithium salt of heparin (lithium heparin) as an anticoagulant are usually marked with green stickers and green tops. Heparin has the advantage over EDTA as an anticoagulant, as it does not affect levels of ions (such as calcium). Heparin can interfere with some immunoassays, however. As lithium heparin is usually used, a person's lithium levels cannot be obtained from these tubes; for this purpose, royal-blue topped Vacutainers containing sodium heparin are used.
- Heparin-coated blood oxygenators are available for use in heart-lung machines. Among other things, these specialized oxygenators are though to improve overall biocompatibility and host homeostasis by providing characteristics similar to native endothelium.
- Common diagnostic procedures require PCR amplification of a patient's DNA, which is easily extracted from white blood cells treated with heparin. This poses a potential problem, since heparin may be extracted along with the DNA, and it has been found to interfere with the PCR reaction at levels as low as 0.002 U in a 50 μL reaction mixture.[11]
References
- ^ Linhardt, R. J., Gunay, N. S. (1999). "Production and Chemical Processing of Low Molecular Weight Heparins". Sem. Thromb. Hem. 3: 5-16.
- ^ Ferro, D., Provasoli, A., et al (1990). "Conformer populations of L-iduronic acid residues in glycosaminoglycan sequences". Carbohydr. Res. 195: 157-167.
- ^ Mulloy, B., Forster, M. J., Jones, C., Davies, D. B. (1993). "NMR and molecular-modelling studies of the solution conformation of heparin". Biochem. J. 293: 849-858.
- ^ Linhardt, R. J. (1991). "Heparin: An important drug enters its seventh decade". Chem. Indust. 2: 45-50.
- ^ Kusmer, Ken. "3rd Ind. preemie infant dies of overdose", Houston Chronicle (Associated Press), 2006.
- ^ {Guyton & Hall, 11 ed.}
- ^ Bjork, I. Lindahl, U. (1982). "Mechanism of the anticoagulant action of heparin". Mol. Cell. Biochem. 48: 161-182.
- ^ Petitou, M., Herault, J. P., Bernat, A., Driguez, P. A., Duchaussoy, P., Lormeau, J. C., Herbert, J. M. (1999). "Synthesis of Thrombin inhibiting Heparin mimetics without side effects". Nature 398: 417-422.
- ^ Kojima, T. Leone, C. W. et al (1992). "Isolation and characterisation of heparan sulfate proteoglycans produced by cloned rat microvascular endothelial cells". J. Biol. Chem. 267: 4859-4569.
- ^ Chuang, W. Christ, M. D. Peng, J. Rabenstein D. L. (2000). "An NMR and molecular modeling study of the site specific binding of histamine by heparin, chemically modified heparin and heparin derived oligosacchrides". Biochemistry. 39: 3542-3555.
- ^ Yokota, M. Tatsumi, N. Nathalang, O. Yamada, T. Tsuda, I. (1999). "Effects of Heparin on Polymerase Chain Reaction for Blood White Cells". J. Clin. Lab. Anal. 13: 133-140.




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xxx
216.73.xxx.xxx
 Server Time:
Server Time: