 |
|
|
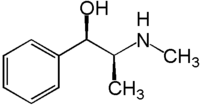
Ephedrine
|
|
| Systematic (IUPAC) name | |
| (1R,2S)-2-(methylamino)-1-phenylpropan-1-ol | |
| Identifiers | |
| CAS number | 299-42-3 |
| ATC code | R01AA03 R03CA02 S01FB02 |
| PubChem | 5032 |
| DrugBank | nil |
| Chemical data | |
| Formula | C10H15NO |
| Mol. weight | 165.23 |
| Pharmacokinetic data | |
| Bioavailability | 85% |
| Metabolism | minimal hepatic |
| Half life | 3–6 hours |
| Excretion | 22-99% renal |
| Therapeutic considerations | |
| Pregnancy cat. | A(AU) A(US) |
| Legal status | S4(AU) Schedule VI(CA) P(UK) |
| Routes | oral, IV, IM, SC |
Ephedrine (EPH) is a sympathomimetic amine similar in structure to the synthetic derivatives amphetamine and methamphetamine. Ephedrine is commonly used as a stimulant, appetite suppressant, concentration aid, decongestant and to treat hypotension associated with regional anaesthesia. Chemically, it is an alkaloid derived from various plants in the genus Ephedra (family Ephedraceae). It is most usually marketed in the hydrochloride and sulfate forms.
In traditional Chinese medicine, the herb ma huang (Ephedra sinica) contains ephedrine as its principal active constituent. The same is true of other herbal products containing extracts from Ephedra species. Nagayoshi Nagai was the first one to isolate ephedrine from Ephedra vulgaris in 1885. The substance called soma mentioned in old Hindu books such as the Rig Veda, may have been ephedra extract.
Contents |
Chemistry
Ephedrine exhibits optical isomerism and has two chiral centres. By convention the enantiomers with opposite stereochemistry around the chiral centres are designated ephedrine, while pseudoephedrine has same stereochemistry around the chiral carbons. That is, (1R,2R)- and (1S,2S)-enantiomers are designated pseudoephedrine; while (1R,2S)- and (1S,2R)-enantiomers are designated ephedrine.
The isomer which is marketed is (-)-(1R,2S)-ephedrine. [1]
As with other phenylethylamines, it is also somewhat chemically similar to methamphetamine, although the amphetamines are more potent and have additional biological effects.
Ephedrine may also be referred to as: (αR)-α-[(1S)-1-(methylamino)ethyl]benzenemethanol, α-[1-(methylamino)ethyl]benzyl alcohol, or L-erythro-2-(methylamino)-1-phenylpropan-1-ol. Ephedrine hydrochloride has a melting point of 187-188°C. [2]
Mode of action
Ephedrine is a sympathomimetic amine - that is, its principal mechanism of action relies on its indirect action on the adrenergic receptor system, which is part of the sympathetic nervous system or SNS. Whilst it may have weak agonist activity at α- and β-adrenergic receptors, the principal mechanism is to displace noradrenaline from storage vesicles in presynaptic neurons. The displaced noradrenaline is released into the neuronal synapse where it is free to activate the postsynaptic adrenergic receptors.
Ephedrine's mechanism of action on neurotransmission in the brain is wide. Its action as an agonist at most major norepinephrine receptors and its ability to induce moderate stimulation of the release of both dopamine and to a lesser extent, serotonin, is presumed to have a major role in its mechanism of action.
Because of ephedrine's ability to potentiate dopamine neurotransmission it is thought to have addictive properties by some researchers.
While ephedrine's role in the serotonin system is less understood there is preliminary documentation of clinically significant agonism at excitory serotonin receptors, perhaps as a downstream response to the large release of norepinephrine in the nucleus accumbens (commonly referred to as the "pleasure center" of the brain). In mice, stereotypical behaviour was both easily induced by administration of ephedrine and it's primary alkaloids and reversed when serotonin antagonists were administered.
Clinical use

Indications
Ephedrine was once widely used as a topical decongestant and as a bronchodilator in the treatment for asthma. It continues to be used for these indications, although its popularity is waning due to the availability of more effective agents for these indications which exhibit fewer adverse effects [3]. The role in nasal congestion has largely been replaced by more potent α-adrenergic receptor agonists (e.g. oxymetazoline). Similarly the role of ephedrine in asthma has been almost entirely replaced by β2-adrenergic receptor agonists (e.g. salbutamol). Ephedrine continues to be used intravenously in the reversal of hypotension from spinal/epidural anaesthesia [3]. It is also used in other hypotensive states, including overdose with ganglionic blocking agents, antiadrenergic agents, or other medications that lower blood pressure [4]. It can be used in narcolepsy and nocturnal enuresis.
In traditional Chinese medicine, ephedrine has been used in the treatment of asthma and bronchitis for centuries [5].
An ECA stack is a component found in thermogenic weight loss pills, composed of ephedrine, caffeine and aspirin working to speed up the metabolism and thus cause food energy to burn faster. The ECA stack is a popular supplement taken by body builders before workouts due to the increased amount of energy and alertness.
For many years, the US Coast Guard recommended ephedrine together with an equal 25 mg dose of promethazine to its sailors to combat seasickness. Promethazine manages nausea and ephedrine fights the ensuing drowsiness. Commonly referred to as the Coast Guard cocktail, ephedrine may still be available for prescription for this purpose.
Adverse effects
Adverse drug reactions (ADRs) are more common with systemic administration (e.g. injection or oral administration) compared to topical administration (e.g. nasal instillations). ADRs associated with ephedrine therapy include [3]:
- Cardiovascular: tachycardia, cardiac arrhythmias,
angina pain, vasoconstriction with hypertension
Dermatological: flushing,acne vulgaris
Gastrointestinal: nausea, appetite loss, anorexia
Genitourinary: increased urine output due to increased blood flow
Nervous system:restlessness, confusion, insomnia, mild euphoria, mania/hallucinations (rare except in previously existing psychiatric conditions), delusions, formication (may be possible, but lacks documented evidence) paranoia, hostility, confusion, panic, stereotypical behaviour ('obsessive compulsive' or repetitive tasks such as cleaning, grooming, organizing items arbitrarily) agitation
Respiratory: dyspnea, pulmonary edema
Miscellaneous: dizziness, headache, tremor, hyperglycemic reactions
The approved maximum daily dosage of ephedrine for use as a bronchodilator is 150mg, as specified on the packaging of the bronchodilator and expectorant combination, Bronkaid, made by Bayer pharmaceuticals.
Overdose can lead to death, although the approved dose is not likely to cause severe reactions when used as directed.
Ephedrine can also lead to damage of the brain receptors over a period of large usage this is because of its constant action on the nuerochemicals. It also leads to high increase in blood pressure which over time can lead to damage in the blood vesasals.
Contraindications
Ephedrine should not be used in conjunction with certain antidepressants, namely SNRIs (Selective norepinephrine re-uptake inhibitors), as this increases the risk of the above symptoms due to excessive serum levels of norepinephrine.
Wellbutrin is an example of an antidepressant with an amphetamine-like structure similar to ephedrine. It has a similar action but also releases serotonin from presynaptic clefts. It should not be used with ephedrine as it may increase the liklihood of the above side effects.
Ephedrine should be used in caution in patients with inadequate fluid replacement, impaired adrenal function, hypoxia, hypercapnia, acidosis, hypertension, hyperthyroidism, prostatic hypertrophy, diabetes mellitus, cardiovascular disease, during delivery if maternal BP > 130/80 mmHg, and lactation.[6]
Contraindications for the use of ephedrine include: closed angle glaucoma, phaeochromocytoma, asymmetric septal hypertrophy (idiopathic hypertrophic subaortic stenosis), concomitant or recent (previous 14 days) monoamine oxidase inhibitor (MAOI) therapy, general anaesthesia with halogenated hydrocarbons (particularly cyclopropane or halothane), tachyarrhythmias or ventricular fibrillation, hypersensitivity to ephedrine or other stimulants, and psychoneurosis (bipolar disorder, severe depression, obsessive compulsive disorder, etc.)
Ephedrine should NOT be used at any time during pregnancy unless specifically indicated by a qualified physician and ONLY when other options are unavailable.[6.
Recreational and illicit use

Anecdotal reports have suggested that ephedrine helps studying, thinking, or concentrating to a greater extent than caffeine. Some students and some white-collar workers have used ephedrine (or Ephedra-containing herbal supplements) for this purpose, as well as some professional athletes and weightlifters. It is common for many athletes to use stimulants while exercising. Such misuse of ephedrine has been associated with stimulant dependence, as well as deaths from heatstroke in athletes and circulatory problems such as aortic aneurysm in weightlifters.
As a phenylethylamine, ephedrine has a similar chemical structure to amphetamines. Ephedrine can be used in the synthesis of methamphetamine by chemical reduction; this has made ephedrine a highly sought-after chemical precursor in the illicit manufacture of methamphetamine. The most popular method for reducing ephedrine to methamphetamine is similar to the Birch reduction, in that it uses anhydrous ammonia and lithium metal in the reaction. The second most popular method uses red phosphorus, iodine, and ephedrine in the reaction.
Through oxidation, ephedrine can be easily synthesized into methcathinone. Ephedrine is listed as a Table I precursor under the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances [7].
Ephedrine has been reported to cause both physical and psychological dependence after excessive long-term use. This is particularly true with oral forms of ephedrine, since parenteral administration is unlikely to occur over long periods [6].
Neurotoxicity of Ephedrine
As a sympathomimetic agent similar in structure and activity to amphetamines, there has been a dispute over whether ephedrine produces some of the same neurodegenerative effects. It has been shown clinically that certain amphetamines (namely (d)-amphetamine and (d)-methamphetamine) can cause varying levels of long-term dopamine depletion in dopamine-rich brain and nervous centers such as the putamen and the basal ganglia.
Several studies have recently compared the quantities of such neurotransmitters as serotonin, dopamine, glutamate, and adrenaline after concurrent administration of ephedrine and various amphetamine-like agents. The results showed that ephedrine has a reduced neurotoxic effect on dopamine than its amphetamine counterparts.
Ephedrine increases serum dopamine levels minimally in comparison with an equivalent dose of dextroamphetamine (Adderall®). Dextromethamphetamine (Desoxyn®) raises dopamine levels dramatically (more than two times that of an equivalent dose of dextroamphetamine). This supports the general consensus that ephedrine has more of a peripheral action on the sympathetic nervous system, whereas amphetamines appear to cross the blood brain barrier more freely and tend to have a stronger central action. The fact that dopamine is believed to play a major role in the addiction response has been used in recent years as justification for controlling the distribution of dextroamphetamine and dextromethamphetamine, along with various other amphetamines.[8]
Legality in USA
Ephedrine itself has never been illegal. In 1996, the FDA proposed a regulation on ephedra (the herb from which ephedrine is obtained), which limited an ephedra dose to 8mg with no more than 24mg per day. This proposed rule was then withdrawn in 2000 because "concerns regarding the agency's basis for proposing a certain dietary ingredient level and a duration of use limit for these products." In 2004, the FDA created a ban on ephedrine alkaloids that are marketed for reasons other than asthma, colds, allergies, other disease, or traditional Asian use. Products such as VasoPro's ephedrine HCL contains Guaifenesin, which thins the mucus in the air passages and makes it easier to clear the airway, and thus is sold as a bronchodilator. On April 14th, 2005, the US Federal District Court ruled that the FDA did not have proper evidence that low dosages of ephedrine alkaloids are actually unsafe, but on August 17, 2006, the U.S. Court of Appeals for the Tenth Circuit in Denver upheld the FDA's final rule declaring all dietary supplements containing ephedrine alkaloids adulterated, and therefore illegal for marketing in the United States. Ephedrine is, however, still legal in many applications outside of as dietary supplements. However, currently purchasing is limited and records are kept of how much ephedrine is purchased, with the specifics varying from state to state. Although the FDA claims that their ruling is a result from several deaths from people taking large doses, some people believe that it is only because ephedrine is one of the key ingredients for creating crystal meth, and that is the reason the FDA limits the total quantity one can purchase.
Footnotes
- ^ (1989) Edited by Reynolds JEF Martindale: The complete drug reference, 29th edition, London: Pharmaceutical Press. ISBN 0-85369-210-6.
- ^ Budavari S, editor. The Merck Index: An encyclopedia of chemicals, drugs, and biologicals, 12th edition. Whitehouse Station: Merck
- ^ a b c Joint Formulary Committee. British National Formulary, 47th edition. London: British Medical Association and Royal Pharmaceutical Society of Great Britain; 2004. ISBN 0-85369-854-9
- ^ Bicopoulos D, editor. AusDI: Drug information for the healthcare professional, 2nd edition. Castle Hill: Pharmaceutical Care Information Services; 2002.
- ^ Ford MD, Delaney KA, Ling LJ, Erickson T, editors. Clinical Toxicology. Philadelphia: WB Saunders; 2001. ISBN 0-72165-485-1 Research Laboratories; 1996. ISBN 0-91191-012-3
- ^ a b c Mayne Pharma. Ephedrine sulfate injection DBL (Approved Product Information). Melbourne: Mayne Pharma; 2004
- ^ http://www.incb.org/pdf/e/list/red.pdf
- ^ Txsci.oxfordjournals




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xxx
216.73.xxx.xxx
 Server Time:
Server Time: