| Dopamine | |
|---|---|
 |
|
| General | |
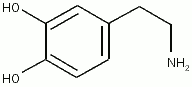
| Systematic name | 4-(2-aminoethyl)benzene-1,2-diol |
| Other names | 2-(3,4-dihydroxyphenyl)ethylamine; 3,4-dihydroxyphenethylamine; 3-hydroxytyramine; DA; Intropin Revivan; Oxytyramine |
| Molecular formula | C8H11NO2 |
| SMILES | C1=CC(=C(C=C1CCN)O)O |
| Molar mass | 153.178 g/mol |
| Appearance | white powder with distinctive smell |
| CAS number | [51-61-6] |
| Properties | |
| Solubility in water | 60.0 g/100 ml (? °C), solid |
| Melting point | 128 °C (401 K) |
| Hazards | |
| R/S statement |
R: 36/37/38 S: 26-36 |
| RTECS number | UX1088000 |
|
Except where noted otherwise, data are given
for materials in their standard state (at 25 °C, 100 kPa) |
|
Dopamine is a chemical naturally produced in the body. In the brain, dopamine functions as a neurotransmitter, activating dopamine receptors. Dopamine is also a neurohormone released by the hypothalamus. Its main function as a hormone is to inhibit the release of prolactin from the anterior lobe of the pituitary.
Dopamine can be supplied as a medication that acts on the sympathetic nervous system, producing effects such as increased heart rate and blood pressure. However, since dopamine cannot cross the blood-brain barrier, dopamine given as a drug does not directly affect the central nervous system. To increase the amount of dopamine in the brains of patients with diseases such as Parkinson's disease and Dopa-Responsive Dystonia, a synthetic precursor to dopamine such as L-DOPA can be given, since this will cross the blood-brain barrier.
Contents |
Biochemistry
Dopamine has the chemical formula (C6H3(OH)2-CH2-CH2-NH2). Its chemical name is 4-(2-aminoethyl)benzene-1,2-diol and it is abbreviated "DA."
As a member of the catecholamine family, dopamine is a precursor to epinephrine (adrenaline) and norepinephrine (noradrenaline) in the biosynthetic pathways for these neurotransmitters. Arvid Carlsson won a share of the 2000 Nobel Prize in Physiology or Medicine for showing that dopamine is not just a precursor to these, but a neurotransmitter as well.
Dopamine is synthesized in the body (mainly by nervous tissue and adrenal glands) first by the hydration of the amino acid tyrosine to DOPA by tyrosine hydroxylase and then by the decarboxylation of DOPA by aromatic-L-amino-acid decarboxylase. In neurons, dopamine is packaged after synthesis into vesicles, which are then released in response to the presynaptic action potential. The inactivation mechanism of neurotransmission are 1) uptake via a specific transporter; 2) enzymatic breakdown; and 3) diffusion. Uptake back to the presynaptic neuron via the dopamine transporter is the major role in the inactivation of dopamine neurotransmission. The recycled dopamine will face either breakdown by an enzyme or be re-packaged into vesicles and reused.
Functions in the brain
Dopamine has many functions in the brain. Most importantly, dopamine is central to the reward system[1]. Dopamine neurons may have the role to emit a teaching signal for prioritizing and learning of reward-directed behaviour and to code reward information relative to established predictions.
Movement
Dopamine affects the basal ganglia motor loop which in turn affects the way the brain controls our movements. Shortage of dopamine, particularly the death of dopamine neurons in the nigrostriatal pathway, causes Parkinson's disease, in which a person loses the ability to execute smooth, controlled movements.
Cognition and frontal cortex
In the frontal lobes, dopamine controls the flow of information from other areas of the brain. Dopamine disorders in this region of the brain can cause a decline in neurocognitive functions, especially memory, attention and problem-solving. Reduced dopamine concentrations in the prefrontal cortex are thought to contribute to attention deficit disorder and negative schizophrenia. Conversely, anti-psychotic medications rely on their inhibition of dopamine uptake and are used in the treatment of positive symptoms in schizophrenia.
Regulating prolactin secretion
Dopamine is the primary neuroendocrine regulator of the secretion of prolactin from the anterior pituitary gland. Dopamine produced by neurons in the arcuate nucleus of the hypothalamus is secreted into the hypothalamo-hypophysial blood vessels of the median eminence, which supply the pituitary gland. The lactotrope cells that produce prolactin, in the absence of dopamine, secrete prolactin continuously; dopamine inhibits this secretion.
Motivation and pleasure
Dopamine is commonly associated with the pleasure system of the brain, providing feelings of enjoyment and reinforcement to motivate a person proactively to perform certain activities. Dopamine is released (particularly in areas such as the nucleus accumbens and striatum) by naturally rewarding experiences such as food, sex, use of certain drugs and neutral stimuli that become associated with them. This theory is often discussed in terms of drugs (such as cocaine and amphetamines), which seem to be directly or indirectly related to the increase of dopamine in these areas, and in relation to neurobiological theories of chemical addiction, arguing that these dopamine pathways are pathologically altered in addicted persons. However, cocaine and amphetamine influence separate mechanisms of action.
Cocaine is a dopamine transporter blocker that competitively inhibits dopamine uptake to increase the lifetime of dopamine and augments an overabundance of dopamine (an increase of up to 150%) within the parameters of the dopamine neurotransmitters. Like cocaine, amphetamines increase the concentration of dopamine in the synaptic gap, but by a different mechanism. Amphetamines are similar in structure to dopamine, and so can enter the terminal button of the presynaptic neuron via its dopamine transporters as well as by diffusing through the neural membrane directly. When entering inside the presynaptic neuron, amphetamines force the dopamine molecules out of their storage vesicles and expel them into the synaptic gap by making the dopamine transporters work in reverse. Dopamine's role in experiencing pleasure has been questioned by several researchers. It has been argued that dopamine is more associated with anticipatory desire and motivation (commonly referred to as "wanting") as opposed to actual consummatory pleasure (commonly referred to as "liking"). Dopamine is released when unpleasant or aversive stimuli are encountered, and so motivates towards the pleasure of avoiding or removing the unpleasant stimuli.
Recent research suggests that the firing of dopamine neurons is a motivational chemical as a result of reward-anticipation. This is based on evidence that, when a reward is perceived to be greater than expected, the firing of certain dopamine neurons increases, which correspondingly increases desire or motivation toward the reward.
Clues to dopamine's role in motivation, desire and pleasure have come from studies performed on animals. In one such study rats were depleted of dopamine by up to 99% in the nucleus accumbens and neostriatum using 6-hydroxydopamine.[1] With this large reduction in dopamine, the rats would no longer eat by their own volition. The researchers then force fed the rats food and noted whether they had the proper facial expressions indicating whether they liked or disliked it. The researchers of this study concluded that the reduction in dopamine did not reduce the rat's consummatory pleasure, only the desire to actually eat. In another study, mutant hyperdopaminergic (increased dopamine) mice show higher "wanting" but not "liking" of sweet rewards.[2]
In humans, though, drugs that reduce dopamine activity (e.g., antipsychotics) have been shown to reduce motivation as well as cause anhedonia (the inability to experience pleasure).[3] Conversely the selective D2/D3 agonists pramipexole and ropinirole have anti-anhedonic properties as measured by the Snaith-Hamilton Pleasure Scale.[4] (The Snaith-Hamilton-Pleasure-Scale (SHAPS), introduced in English in 1995, assesses self-reported anhedonia in psychiatric patients.)
Opioid and cannabinoid transmission instead of dopamine may modulate consummatory pleasure and food palatability(liking).[5] This could explain why animals "liking" of food is independent of brain dopamine concentration. Other consummatory pleasures, however, may be more associated with dopamine. One study found that both anticipatory and consummatory measures of sexual behavior (male rats) were disrupted by DA receptor antagonists.[6] Libido can be increased by drugs that affect dopamine but not by drugs that affect opioid peptides or other neurotransmitters.
Sociability is also closely tied to dopamine neurotransmission. Low D2 receptor binding is found in people with social anxiety. Traits common to negative schizophrenia (social withdrawal, apathy, anhedonia) are thought to be related to a hypodopaminergic state in certain areas of the brain. In instances of bipolar, manic subjects can become hypersocial as well as hypersexual. This is also credited to an increase in dopamine, because mania alleviates from dopamine blocking antipsychotics.
Other theories reinforce that the crucial role of dopamine may be in desire, or anticipating pleasurable activity. Related theories [7] argue that dopamine function may be involved in the salience ('noticeableness') of perceived objects and events, with potentially important stimuli such as: 1) rewarding things or 2) dangerous or threatening things seeming more noticeable or important. This hypothesis argues that dopamine assists decision-making by influencing the priority, or level of desire, of such stimuli to the person concerned.
Pharmacological blockade of brain dopamine receptors increases rather than decreases drug-taking behavior. Since blocking dopamine decreases desire, the increase in drug taking behavior may be seen as not a chemical desire but as a deeply psychological desire to just 'feel something'.
Deficits in dopamine levels are implicated as one of several possible causes for Adult attention-deficit disorder (AADD), and some types of medications used to treat Attention-deficit hyperactivity disorder (ADHD/ADD) will help to stimulate dopaminergic systems, leading to potentially heightened sensation, for those afflicted by it and receiving treatment for it.
Links to psychosis
Disruption to the dopamine system has also been strongly linked to psychosis and schizophrenia.[8] Dopamine neurons in the mesolimbic pathway are particularly associated with these conditions. This is partly due to the discovery of a class of drugs called the phenothiazines (which block D2 dopamine receptors) that can reduce psychotic symptoms, and partly due to the finding that drugs such as amphetamine and cocaine (which are known to greatly increase dopamine levels) can cause psychosis. Because of this, most modern antipsychotic medication is designed to block dopamine function to varying degrees.
Depression
Dopamine is a neurotransmitter that is involved in depression. Amphetamines and dopamine reuptake blockers have potent anti-depressant effects but these drugs quickly lose their benefit after they deplete dopamine levels in the brain. Antidepressants appear to primarily enhance serotonergic neurotransmission during preliminary drug administration but it takes several weeks for the antidepressant effect to be noticed. The late effect of antidepressants is thought to involve the indirect serotonergic modulation of dopaminergic neurotransmission. Blocking the D2 dopamine receptor is known to cause relapse in patients that have achieved remission from depression, and such blocking also counteracts the effectiveness of SSRI medication.
Therapeutic use
Levodopa is a dopamine precursor used to treat Parkinson's disease. It is typically co-administered with an inhibitor of peripheral decarboxylation (DDC, dopa decarboxylase), such as carbidopa or benserazide. Inhibitors of alternative metabolic route for dopamine by catechol-O-methyl transferase are also used. These include entacapone and tolcapone.
Dopamine is also used as an inotropic drug in patients with shock to increase cardiac output and blood pressure.
Major pathways
- Mesocortical pathway
Mesolimbic pathway
Nigrostriatal pathway
Tuberoinfundibular pathway
See also
External links
Footnotes
- ^ Berridge K, Robinson T (1998). "What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience?". Brain Res Brain Res Rev 28 (3): 309-69. PMID 9858756.
- ^ Peciņa S, Cagniard B, Berridge K, Aldridge J, Zhuang X (2003). "Hyperdopaminergic mutant mice have higher "wanting" but not "liking" for sweet rewards.". J Neurosci 23 (28): 9395-402. PMID 14561867.
- ^ Lambert M, Schimmelmann B, Karow A, Naber D (2003). "Subjective well-being and initial dysphoric reaction under antipsychotic drugs - concepts, measurement and clinical relevance.". Pharmacopsychiatry 36 Suppl 3: S181-90. PMID 14677077.
- ^ Lemke M, Brecht H, Koester J, Kraus P, Reichmann H (2005). "Anhedonia, depression, and motor functioning in Parkinson's disease during treatment with pramipexole.". J Neuropsychiatry Clin Neurosci 17 (2): 214-20. PMID 15939976.
- ^ Peciņa S, Berridge K (2005). "Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness?". J Neurosci 25 (50): 11777-86. PMID 16354936.
- ^ Pfaus J, Phillips A (1991). "Role of dopamine in anticipatory and consummatory aspects of sexual behavior in the male rat.". Behav Neurosci 105 (5): 727-43. PMID 1840012.
- ^ Schultz W (2002). "Getting formal with dopamine and reward". Neuron 36 (2): 241-263. PMID 12383780.
- ^ Disruption of gene interaction linked to schizophrenia. St. Jude Children's Research Hospital. Retrieved on 2006-07-06.




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xxx
216.73.xxx.xxx
 Server Time:
Server Time: