 |
|
|
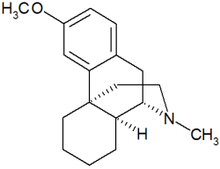
Dextromethorphan
|
|
| Identifiers | |
| CAS number | 125-71-3 |
| ATC code | R05DA09 |
| PubChem | 5360696 |
| DrugBank | APRD00655 |
| Chemical data | |
| Formula | C18H25NO |
| Mol. weight | 271.4 g/mol |
| Pharmacokinetic data | |
| Metabolism | Hepatic (CYP2D6, CYP3A4 and CYP3A5-mediated) |
| Half life | 1.4–3.9 hours |
| Excretion | Renal |
| Therapeutic considerations | |
| Pregnancy cat. | A(AU) C(US) |
| Legal status | S2(AU) |
| Routes | Oral |
Dextromethorphan (DM or DXM) is an antitussive drug that is found in many over-the-counter cold and cough preparations, usually in the form of dextromethorphan hydrobromide. It is also used as a recreational drug.
Contents |
Chemistry
Dextromethorphan is a salt of the methyl ether dextrorotatory isomer of levorphanol, a narcotic (opioid) analgesic (and DXM itself is an enantiomeric isomer, that is, a mirror image in the 3D space, of levomethorphan, a substance considered an opioid[1]). It is chemically named as 3-methoxy-17-methyl-9(alpha), 13(alpha), 14(alpha)-morphinan hydrobromide monohydrate. DXM occurs as white crystals, is sparingly soluble in water, and freely soluble in alcohol. The drug is dextrorotatory in water (at 20 degrees Celsius, Sodium D-line) with a specific rotation of +27.6 degrees.
Indications
The FDA approved dextromethorphan for over-the-counter sale as a cough suppressant in 1958. This filled the need for a cough suppressant lacking the abuse liability and addictive properties of codeine phosphate, the most widely used cough medication at the time. The advantage of dextromethorphan preparations over those containing codeine (now prescription only in the United States) was the lack of physical addiction potential and sedative side-effects, although as with most cough supressants, studies show that its effectiveness is highly debatable. See also: Cough medicine controversy
Pharmacodynamics
At therapeutic doses, the drug acts centrally to elevate the threshold for coughing, without inhibiting ciliary activity. Dextromethorphan is rapidly absorbed from the gastrointestinal tract, and exerts its activity within 15 to 60 minutes of ingestion. The duration of action after oral administration is approximately three to eight hours. Because administration of DXM can be accompanied by histamine release, its use in atopic children is very limited.
The average dosage necessary for effective antitussive therapy is between 10mg and 30mg every four to six hours.
According to the WHO committee on Drug Dependence, dextromethorphan, when used recreationally (see non-medical use of dextromethorphan), does not produce physical addiction but can generate slight psychological dependence in some users.
Clinical pharmacology
Following oral administration, dextromethorphan is rapidly absorbed from the gastrointestinal tract, where it enters the bloodstream and crosses the blood-brain barrier. The first-pass through the hepatic portal vein results in some of the drug being metabolized into an active metabolite of dextromethorphan, dextrorphan, the 3-hydroxy derivative of dextromethorphan. The therapeutic activity of dextromethorphan is believed to be caused by both the drug and this metabolite. Dextromethorphan is metabolized by various liver enzymes and subsequently undergoes O-demethylation (producing dextrorphan), N-demethylation, and partial conjugation with glucuronic acid and sulfate ions. Hours after dextromethorphan therapy, (in humans) the metabolites (+)-3-hydroxy-N-methylmorphinan, (+)-3-morphinan, and traces of the unchanged drug are detectable in the urine.
One well known metabolic catalyst involved is a specific cytochrome P450 enzyme known as 2D6, or CYP2D6. A significant portion of the population has a functional deficiency in this enzyme (and are known as poor CYP2D6 metabolizers). As CYP2D6 is the primary metabolic pathway in the inactivation of dextromethorphan, the duration of action and effects of dextromethorphan are significantly increased in such poor metabolizers. Deaths and hospitalizations have been reported in recreational use by poor CYP2D6 metabolizers.
A large number of medications (including antidepressants) are potent inhibitors of CYP2D6 (see CYP2D6 article). There exists, therefore, the potential of drug-drug interactions between dextromethorphan and concomitant medications. There have been reports of fatal consequences arising from such interactions.[2]
Dextromethorphan crosses the blood-brain barrier, and the following pharmacological actions have been reported:
- NMDA glutamatergic receptor antagonist
- Dopamine reuptake inhibitor[3]
- σ1 and σ2 receptor agonist (Zhou & Musacchio, 1991)
- α3β4 nicotinic receptor antagonist[4]
- Serotonin reuptake inhibitor[5]
History
Dextromethorphan was first patented with U.S. Patent 2,676,177, and was approved for over-the-counter purchase as an antitussive in 1958.
During the 1960s and 1970s, DXM became available in an over-the-counter tablet form by the brand name Romilar. It was put on the shelves in hopes of cutting down on codeine cough remedies. In 1973, Romilar was taken off the shelves after a burst in sales due to common recreational use. It was then replaced by cough syrup, in an attempt to cut down on recreational usage.
Fibromyalgia treatment
Dextromethorphan is currently being investigated as a potential treatment for fibromyalgia symptoms.
Recreational use
Since their introduction, preparations containing the over-the-counter drug dextromethorphan have been used in a manner inconsistent with their labeling, often as a recreational drug or to induce intoxication (sometimes referred to as "robo-tripping"). Dextromethorphan has little to no psychological effect in the doses used medically, however alteration of consciousness generally occurs following ingestion of approximately 7 to 50 times the therapeutic dose over a relatively short period of time. [2]
People who study the specific effects of psychotropic substances classify DXM as a dissociative drug, a major subclass of hallucinogenic drugs, along with Ketamine and Phencyclidine. It generally does not produce withdrawal symptoms characteristic of physically addictive substances, but psychological addiction has been reported by some users.
DXM, when consumed in low recreational doses (usually under 200mg), is often described as having a buoyant, vaguely psychedelic effect similar to a mixture of alcohol, opiates, and marijuana. With higher doses, intense euphoria and vivid imagination may occur as bizarre feelings of dissociation increase. With very high doses, profound alterations in consciousness have been noted, and users often report out of body experiences or temporary psychosis. One of the unique features of a high dose DXM trip is the ability to relive past memories. Most users find such high doses to be extremely uncomfortable and most are unwilling to repeat it. Flanging (speeding up or slowing down) of sensory input also occurs, which is another unique feature of high dose DXM trips. In 1981, a paper by Gosselin estimated the lethal dose between 50 and 500 mg/kg.
Individual reactions to recreational doses of Dextromethorphan vary widely. Some find the effects of the drug to be immensely pleasurable, similar to a combination of opiates and hallucinogens, while others find that the drug produces dysphoria, panic, or dread.
Physical side effects that can occur after ingestion of recreational doses of DXM include a blotchy skin rash, itching (sometimes referred to as "robo itch," short for "Robitussin itch"), and sweating. Many people vomit from recreational doses or feel ill for the first part of the “trip”. When taken in higher doses, physical side effects can include dilated pupils, difficulty urinating, increased urination frequency, extreme diarrhea, fever, tachycardia, loss of appetite, shakiness, seizures, and possible coma and death (however, in pure DXM, this has only been reported when doses exceed 2,000 mg).
See also
Footnotes
- ^ Shulgin, Alexander (2003). DXM (Dextromethorphan). Ask Dr. Shulgin Online. Retrieved on 2006-05-31.
- ^ a b Jones K, Taranto M (2006). "Illicit Drug Manual: Dextromethorphan ("Robo-tripping")". collegehealth-e 1 (4): 13-17.
- ^ http://www.nhtsa.dot.gov/people/injury/research/job185drugs/dextromethorphan.htm
- ^ http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list uids=10869398
- ^ http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list uids=1636059
External links
- Dextroverse
- Dextromethorphan FAQ at third-plateau.org
- Erowid
- Link page to external chemical sources.




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xxx
216.73.xxx.xxx
 Server Time:
Server Time: