 |
|
|
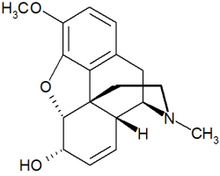
Codeine
|
|
| Systematic (IUPAC) name | |
|
7,8-didehydro-4,5-epoxy- 3-methoxy-17-methylmorphinan-6-ol |
|
| Identifiers | |
| CAS number | 76-57-3 |
| ATC code | R05DA04 |
| PubChem | 5284371 |
| DrugBank | APRD00120 |
| Chemical data | |
| Formula | C18H21NO3 |
| Mol. weight | 299.364 g/mol |
| Pharmacokinetic data | |
| Bioavailability | well absorbed |
| Metabolism | Hepatic |
| Half life | 2–4 hours |
| Excretion | renal |
| Therapeutic considerations | |
| Pregnancy cat. | A(AU) |
| Legal status | S8(AU) Schedule I(CA) Class B(UK) Schedule II(US) |
| Routes | oral, intra-nasally, intra-rectally, SC, IM |
Codeine (INN) or methylmorphine is an opiate used for its analgesic, antitussive and antidiarrheal properties. It is marketed as the salts codeine sulfate and codeine phosphate. Codeine hydrochloride is more commonly marketed in continental Europe and other regions.
Codeine is an alkaloid found in opium in concentrations ranging from 0.3 to 3.0 percent. While codeine can be extracted from opium, most codeine is synthesized from morphine through the process of O-methylation.
Contents |
Indications
Approved indications for codeine include:
- Cough, though its efficacy has been disputed.[1]
- Diarrhea
- Moderate to severe pain
Codeine is sometimes marketed in combination preparations with paracetamol (acetaminophen) as co-codamol (best known as Tylenol 3), with aspirin co-codaprin or with ibuprofen. These combinations provide greater pain relief than either agent used alone (q.v. Drug Synergy).
Controlled substance
In the United States, codeine is regulated by the Controlled Substances Act. It is a Schedule II controlled substance for pain-relief products containing codeine alone. In combination with aspirin or acetaminophen (paracetamol/tylenol) it is listed as Schedule III. Codeine is also available outside the United States as an over-the-counter drug in liquid cough-relief formulations. Internationally, codeine is a Schedule II drug under the Single Convention on Narcotic Drugs.[2]
In the United Kingdom, codeine is regulated by the Misuse of Drugs Act 1971; it is a Class B drug, except for concentrations of less than 8 mg when combined with paracetamol, or 12.5 mg when combined with ibuprofen, which are available in many over the counter preparations.
In Australia, New Zealand and Canada, codeine is regulated; however, it is available without prescription in combination preparations from licensed pharmacists in doses up to 15 mg/tablet (8 mg/tablet in Canada).
In Canada, codeine can only be sold in combination with 2 or more ingredients, which has resulted in the prevalence of AC&C (aspirin, codeine, and caffeine), and similar combinations using acetaminophen rather than asprin. Caffeine, being a stimulant, tends to offset the sedative effects of codeine.
Pharmacokinetics
Codeine is considered a prodrug, since it is metabolised in vivo to the principal active analgesic agent morphine. It is, however, less potent than morphine since only about 10% of the codeine is converted. It also has a correspondingly lower dependence-liability than morphine.
Theoretically, a dose of approximately 200 mg (oral) of codeine must be administered to give equivalent analgesia to 30 mg (oral) of morphine (Rossi, 2004). It is not used, however, in single doses of greater than 60mg (and no more than 240 mg in 24 hours) since there is a ceiling effect.
The conversion of codeine to morphine occurs in the liver and is catalysed by the cytochrome P450 enzyme CYP2D6. Approximately 6–10% of the Caucasian population have poorly functional CYP2D6 and codeine is virtually ineffective for analgesia in these patients (Rossi, 2004). Many of the adverse effects, however, are still experienced. Also, some medications are CYP2D6 inhibitors and reduce or even completely eliminate the efficacy of codeine. The most notorious of these are the selective serotonin reuptake inhibitors, such as fluoxetine (Prozac) and citalopram (Celexa).
Pharmacology
Codeine itself has weak affinity for the μ-opioid receptor. Its principal analgesic actions are mediated by the affinity of morphine for the μ-opioid receptor, though other therapeutic and adverse effects are produced by activation of other opioid receptors.
Adverse effects
Common adverse drug reactions associated with the use of codeine include itching, nausea, vomiting, drowsiness, dry mouth, miosis, orthostatic hypotension, urinary retention and constipation.[3]
Tolerance to many of the effects of codeine develops with prolonged use, including therapeutic effects. The rate at which this occurs develops at different rates for different effects, with tolerance to the constipation-inducing effects developing particularly slowly for instance.
A potentially serious adverse drug reaction, as with other opioids, is respiratory depression. This depression is dose-related and is the mechanism for the potentially fatal consequences of overdose.
Another side effect commonly noticed is the lack of sexual drive. It is generally due to lethargy caused by abuse of codeine.
Codeine has also been known to interact negatively with some psychiatric medications such as reboxetine and venlafaxine.
Recreational use
Codeine is often used as a recreational drug. This may be due to its easy availability over the counter or on prescription in combination products (which, in certain countries, are scheduled lower than codeine as a single-agent). People use it in order to obtain the euphoric effects associated with use of opioids. Codeine-containing cough syrups are often taken whole by drinking the syrup; combination pills may be taken whole or crushed and insufflated (snorted), or the codeine may be extracted using methods like cold water extraction.
- In certain areas of the United States, such as Texas, codeine in syrup form is called Lean. It is commonly mixed with alcohol, or into a joint (marijuana cigarette) and smoked.
- When prepared with promethazine, codeine is a Schedule V controlled substance in the United States. In a few states, such as New Jersey, codeine with promethazine is available over the counter. The syrup, sometimes known as Phenergan With Codeine, contains 6.25 mg of promethazine and 10 mg of codeine per teaspoonful.
- In some countries, cough syrups and tablets containing codeine are available without prescription; people will frequently purchase it from multiple pharmacies so as not to incur suspicion. It is reported that in France, 95% of the consumption of Néo-codion cough preparation, containing codeine, cannot be attributed to medical use, but is rather used as a substitute for heroin. A heroin addict may use codeine to ward off the effects of a withdrawal.[4]
- In the United Kingdom, people purchase tablets which combine codeine and paracetamol (acetaminophen), and consume these at higher-than-recommended doses, without apparent concern of the hepatotoxicity associated with large doses of paracetamol. Some try to extract the codeine from the paracetamol through various methods, the most common and simplest being the cold water extraction.
- While the combination of codeine with paracetamol at higher-than-recommended doses can possibly cause hepatotoxicity (liver damage), combination with ibuprofen can result in kidney problems/failure and additional stomach pain and nausea, and combination with aspirin can lead to internal hemorrhaging, particularly gastrointestinal hemorrhage.
- In South Asian countries codeine has become a multi-million-dollar market in the form of codeine-phosphate-containing cough syrups which are easily available at chemist shops without a prescription, notable among them being Corex and Phensydryl. Although it is nominally mandatory to have a prescription, it is a highly neglected rule, especially in smaller cities where prescriptions are ignored on a regular basis. Codeine phosphate tablets are also available over the counter, despite being prescription-only as well. They are among the most abused over-the-counter drugs in South Asia (across all sections of its society), and continue to be freely available despite legislations by States to curb their availability to buyers without prescriptions.
- Certain codeine products are encountered on the illicit market, frequently in combination with carisoprodol. Combinations of codeine and glutethimide (Doriden) used to be fairly commonplace, but are almost unheard of today, due to the withdrawal of glutethimide products from the marketplace in the US and almost all other countries.
- Codeine is also demethylated to illicitly synthesize morphine.[5]
Footnotes
- ^ Schroeder K, Fahey T. "Over-the-counter medications for acute cough in children and adults in ambulatory settings.". Cochrane Database Syst Rev: CD001831. DOI:10.1002/14651858.CD001831. PMID 15495019.
- ^ International Narcotics Control Board. List of Narcotic Drugs under International Control (PDF). Retrieved on 2006-05-24.
- ^ Australian Medicines Handbook (2004). Rossi S Australian Medicines Handbook. Adelaide: Australian Medicines Handbook. ISBN 0-9578521-4-2.
- ^ Boekhout van Solinge, Tim [1996]. “7. La politique de soins des années quatre-vingt-dix”, L'héroïne, la cocaïne et le crack en France. Trafic, usage et politique (in French). Amsterdam: CEDRO Centrum voor Drugsonderzoek, Universiteit van Amsterdam, 247-262.
- ^ Hogshire, Jim (June 1999). Pills-A-Go-Go: A Fiendish Investigation into Pill Marketing, Art, History & Consumption. Los Angeles: Feral House, 216-223. ISBN 0922915539.




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xxx
216.73.xxx.xxx
 Server Time:
Server Time: