| Caffeine | |
|---|---|
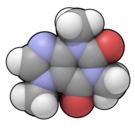 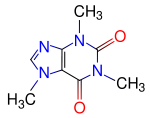 |
|
| General | |
| Systematic name | 1,3,7-trimethyl-1H-purine-2,6(3H,7H)-dione |
| Other names | 1,3,7-trimethylxanthine, trimethylxanthine, theine, mateine, guaranine, methyltheobromine |
| Molecular formula | C8H10N4O2 |
| SMILES | O=C1C2=C(N=CN2C)N(C(=O)N1C)C |
| Molar mass | 194.19 g mol−1 |
| Appearance | Odorless, white needles or powder |
| CAS number | [58-08-2] |
| Properties | |
| Density and phase | 1.2 g/cm³, solid |
| Solubility in water | Slightly soluble |
| Other solvents | Soluble in ethyl acetate, chloroform, pyrimidine, pyrrole, tetrahydrofuran solution; moderately soluble in alcohol, acetone; slightly soluble in petroleum ether, ether, benzene. |
| Melting point | 237 °C |
| Boiling point | 178 °C (sublimes) |
| Acidity (pKa) | 10.4 (40 °C) |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | May be fatal if inhaled, swallowed or absorbed through the skin. |
| NFPA 704 |

1
2
0
|
| Flash point | N/A |
| RTECS number | EV6475000 |
|
Except where noted otherwise, data are given
for materials in their standard state (at 25 °C, 100 kPa) |
|
Caffeine is a xanthine alkaloid compound that acts as a stimulant in humans. Caffeine is sometimes called guaranine when found in guarana, mateine when found in mate, and theine when found in tea. It is found in the leaves and beans of the coffee plant, in tea, yerba mate, and guarana berries, and in small quantities in cocoa, the kola nut and the Yaupon Holly. Overall, caffeine is found in the beans, leaves, and fruit of over 60 plants, where it acts as a natural pesticide that paralyzes and kills certain insects feeding upon them.
Caffeine is a central nervous system (CNS) stimulant, having the effect of temporarily warding off drowsiness and restoring alertness. Beverages containing caffeine, such as coffee, tea, soft drinks and energy drinks enjoy great popularity: caffeine is the world's most widely consumed psychoactive substance. In North America, 90% of adults consume caffeine daily.[1]
Many natural sources of caffeine also contain widely varying mixtures of other xanthine alkaloids, including the cardiac stimulants theophylline and theobromine and other substances such as tannins.
Contents |
Sources

| Caffeine content of select common food and drugs[2][3] | ||
| Product | Serving size | Caffeine per serving (mg) |
|---|---|---|
| Caffeine tablet (Vivarin) | 1 tablet | 200 |
| Excedrin tablet | 1 tablet | 65 |
| Coffee, brewed | 240 mL (8 US fl oz) | 135* |
| Coffee, decaffeinated | 240 mL (8 US fl oz) | 5* |
| Coffee, espresso | 57 mL (2 US fl oz) | 100* |
| Chocolate, Dark (Hershey's Special Dark) | 1 bar (43 g; 1.5 oz) | 31 |
| Chocolate, Milk (Hershey Bar) | 1 bar (43 g; 1.5 oz) | 10 |
| Red Bull | 240 mL (8.2 US fl oz) | 80 |
| Bawls Guarana | 296 mL (10 US fl oz) | 67 |
| Soft drink, Mountain Dew "Dew Fuel" | 355 mL (12 US fl oz) | 54.5 |
| Soft drink, Coca-Cola Classic | 355 mL (12 US fl oz) | 34 |
| Atomic Rush | 255 mL (7 US fl oz) | 100 |
| Tea, green | 240 mL (8 US fl oz) | 15 |
| Tea, leaf or bag | 240 mL (8 US fl oz) | 50 |
| * Estimated average caffeine content per serving. Actual content varies according to preparation. | ||
Caffeine is a plant alkaloid, found in numerous plant species, where it acts as a natural pesticide that paralyzes and kills certain insects feeding upon them.[4] The most commonly used caffeine-containing plants are coffee, tea, and to some extent cocoa. Other, less commonly used, sources of caffeine include the yerba mate[5] and guaraná plants, which are sometimes used in the preparation of teas and energy drinks. Two of caffeine's alternative names, mateine[6] and guaranine,[7] are derived from the names of these plants.
The world's primary source of caffeine is the coffee bean (the seed of the coffee plant), from which coffee is brewed. Caffeine content in coffee varies widely depending on the type of coffee bean and the method of preparation used;[8] even beans within a given bush can show variations in concentration. In general one serving of coffee ranges from about 40 milligrams for a single shot (30 milliliters) of arabica-variety espresso to about 100 milligrams for strong drip coffee. Generally, dark-roast coffee has less caffeine than lighter roasts because the roasting process reduces the bean's caffeine content. Arabica coffee normally contains less caffeine than the robusta variety.[8] Coffee also contains trace amounts of theophylline, but no theobromine.
Tea is another common source of caffeine. Tea usually contains about half as much caffeine per serving as coffee, depending on the strength of the brew. Certain types of tea, such as black and oolong, contain somewhat more caffeine than most other teas. Tea contains small amounts of theobromine and slightly higher levels of theophylline than coffee. Preparation has a significant impact on tea, and color is a very poor indicator of caffeine content.[9] Teas like the green Japanese gyokuro, for example, contain far more caffeine than much darker teas like lapsang souchong, which has very little.
Chocolate derived from cocoa contains a small amount of caffeine. Chocolate is a weak stimulant, which is mostly due to its content of theobromine and theophylline.[10] It contains too little of these compounds for a reasonable serving to create effects in humans that are on par with coffee. A typical 28-gram serving of a milk chocolate bar has about as much caffeine as a cup of decaffeinated coffee.
Caffeine is also a common ingredient of soft drinks such as cola, originally prepared from kola nuts. Soft drinks typically contain about 10 to 50 milligrams of caffeine per serving. By contrast, energy drinks such as Red Bull contain as much as 80 milligrams of caffeine per serving. The caffeine in these drinks either originates from the ingredients used or is an additive derived from the product of decaffeination or from chemical synthesis. Guarana, a prime ingredient of energy drinks, contains large amounts of caffeine with small amounts of theobromine and theophylline in a naturally occurring slow-release excipient.[11]
History of use

Humans have consumed caffeine since the Stone Age.[12] Early peoples found that chewing the seeds, bark, or leaves of certain plants had the effects of easing fatigue, stimulating awareness, and elevating mood. Only much later was it found that the effect of caffeine was increased by steeping such plants in hot water. Many cultures have legends that attribute the discovery of such plants to people living many thousands of years ago.
According to one popular Mongolian legend, the Emperor of China Shennong, reputed to have reigned in about 3,000 BC, accidentally discovered that when some leaves fell into boiling water, a fragrant and restorative drink resulted.[13] Shennong is also mentioned in Lu Yu's Cha Jing, a famous early work on the subject of tea.[14]
The early history of coffee is obscure, but a popular myth traces its discovery to Ethiopia, where Coffea arabica originates. According to this myth, a goatherder named Kaldi observed goats that became elated and sleepless at night after browsing on coffee shrubs and, upon trying the berries that the goats had been eating, experienced the same vitality. The earliest literary mention of coffee may be a reference to Bunchum in the works of the 9th century Persian physician al-Razi. In 1587, Malaye Jaziri compiled a work tracing the history and legal controversies of coffee, entitled "Umdat al safwa fi hill al-qahwa". In this work, Jaziri recorded that one Sheikh, Jamal-al-Din al-Dhabhani, mufti of Aden, was the first to adopt the use of coffee in 1454, and that in the 15th century the Sufis of Yemen routinely used coffee to stay awake during prayers.
Towards the close of the 16th century, the use of coffee was recorded by a European resident in Egypt, and about this time it came into general use in the Near East. The appreciation of coffee as a beverage in Europe, where it was first known as "Arabian wine," dates from the 17th century. During this time "coffee houses" were established, the first being opened in Constantinople and Venice. In Britain, the first coffee houses were opened in London in 1652, at St Michael's Alley, Cornhill. They soon became popular throughout Western Europe, and played a significant role in social relations in the 17th and 18th centuries.[15]
The kola nut, like the coffee berry and tea leaf, appears to have ancient origins. It is chewed in many West African cultures, individually or in a social setting, to restore vitality and ease hunger pangs. In 1911, kola became the focus of one of the earliest documented health scares when the US government seized 40 barrels and 20 kegs of Coca-Cola syrup in Chattanooga, Tennessee, alleging that the caffeine in its drink was "injurious to health".[16] On March 13, 1911, the government initiated The United States vs. Forty Barrels and Twenty Kegs of Coca-Cola, hoping to force Coca-Cola to remove caffeine from its formula by making exaggerated claims, such as that the excessive use of Coca-Cola at one girls' school led to "wild nocturnal freaks, violations of college rules and female proprieties, and even immoralities."[17] Although the judge ruled in favor of Coca-Cola, two bills were introduced to the U.S. House of Representatives in 1912 to amend the Pure Food and Drug Act, adding caffeine to the list of "habit-forming" and "deleterious" substances which must be listed on a product's label.
The earliest evidence of cocoa use comes from residue found in an ancient Mayan pot dated to 600 BC. In the New World, chocolate was consumed in a bitter and spicy drink called xocoatl, often seasoned with vanilla, chile pepper, and achiote. Xocoatl was believed to fight fatigue, a belief that is probably attributable to the theobromine and caffeine content. Chocolate was an important luxury good throughout pre-Columbian Mesoamerica, and cocoa beans were often used as currency.
Chocolate was introduced to Europe by the Spaniards and became a popular beverage by 1700. They also introduced the cacao tree into the West Indies and the Philippines. It was used in alchemical processes, where it was known as Black Bean.
The first coffee house in Europe was opened Paris in the 1800's by an French-Armenian named Pascal. Armenian merchants played in role in the more modern history of coffee and this is the reason why the coffee growing region in is named the Armenia Region of Columbia.
In 1819, the German chemist Friedrich Ferdinand Runge isolated relatively pure caffeine for the first time. According to a legend, he did this at the behest of Johann Wolfgang von Goethe.[18]
Today, global consumption of caffeine has been estimated at 120,000 tons per annum,[19] making it the world's most popular psychoactive substance. This number equates to one serving of a caffeinic beverage for every person, per day. In North America, 90% of adults consume some amount of caffeine daily.
Effects

Caffeine is a central nervous system and metabolic stimulant,[20] and is used both recreationally and medically to reduce physical fatigue and restore mental alertness when unusual weakness or drowsiness occurs. Caffeine stimulates the central nervous system first at the higher levels, resulting in increased alertness and wakefulness, faster and clearer flow of thought, increased focus, and better general body coordination, and later at the spinal cord level at higher doses.[21] The precise amount of caffeine necessary to produce effects varies from person to person depending on body size and degree of tolerance to caffeine. It takes less than an hour for caffeine to begin affecting the body and a mild dose wears off in three to four hours.[21] Consumption of caffeine does not eliminate the need for sleep: it only temporarily reduces the sensation of being tired.
With these effects, caffeine is an ergogenic: increasing the capacity for mental or physical labor. A study conducted in 1979 showed a 7% increase in distance cycled over a period of two hours in subjects who consumed caffeine compared to control tests.[22] Other studies attained much more dramatic results; one particular study of trained runners showed a 44% increase in "race-pace" endurance, as well as a 51% increase in cycling endurance, after a dosage of 9 milligrams of caffeine per kilogram of body weight.[23] The extensive boost shown in the runners is not an isolated case; additional studies have reported similar effects. Another study found 5.5 milligrams of caffeine per kilogram of body mass resulted in subjects cycling 29% longer during high intensity circuits.[24]
Caffeine is sometimes administered in combination with medicines to increase their effectiveness. Caffeine makes pain relievers 40% more effective in relieving headaches and helps the body absorb headache medications more quickly, bringing faster relief.[25] For this reason, many over-the-counter headache drugs include caffeine in their formula. It is also used with ergotamine in the treatment of migraine and cluster headaches as well as to overcome the drowsiness caused by antihistamines.
Breathing problems in premature infants, apnea of prematurity, are sometimes treated with citrated caffeine, which is available only by prescription in many countries.[26] A reduction in bronchopulmonary dysplasia has been exhibited in premature infants treated with caffeine citrate therapy regimens. It is speculated that this reduction in bronchopulmonary dysplasia is tied to a reduction in exposure to positive airway pressure. The only short term risk associated with this treatment is a temporary reduction in weight gain during the therapy.[27]
While relatively safe for humans, caffeine is considerably more toxic to some other animals such as dogs, horses and parrots due to a much poorer ability to metabolize this compound. Caffeine has a much more significant effect on spiders, for example, than most other drugs do.[28]
Overuse

Caffeine is a drug that in large amounts, especially over an extended period of time, can lead to a condition termed "caffeinism." Caffeinism usually combines physical addiction with a wide range of unpleasant physical and mental conditions including nervousness, irritability, anxiety, tremulousness, muscle twitching (hyperreflexia), insomnia, and heart palpitations.[29] (Under a rigid definition of addiction, meaning a process of escalating use, "caffeine dependency" would be a more descriptive term. However, under the widely accepted definition "chronic pattern of behavior that is perceived to be difficult to quit," caffeine may be said to be addictive.) Furthermore, because caffeine increases the production of stomach acid, high usage over time can lead to peptic ulcers, erosive esophagitis, and gastroesophageal reflux disease.[30] However, since both "regular" and decaffeinated coffees have also been shown to stimulate the gastric mucosa and increase stomach acid secretion, caffeine is probably not the only component of coffee responsible.[31]
There are four caffeine-induced psychiatric disorders recognized by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition: caffeine intoxication, caffeine-induced anxiety disorder, caffeine-induced sleep disorder, and caffeine-related disorder not otherwise specified (NOS).
Caffeine intoxication
An acute overdose of caffeine, usually in excess of 250 milligrams (more than 2-3 cups of brewed coffee), can result in a state of central nervous system overstimulation called caffeine intoxication. The symptoms of caffeine intoxication may include restlessness, nervousness, excitement, insomnia, flushing of the face, increased urination, gastrointestinal disturbance, muscle twitching, a rambling flow of thought and speech, irregular or rapid heart beat, and psychomotor agitation.[29][32]
In cases of extreme overdose, death can result. The median lethal dose (LD50) of caffeine is 192 milligrams per kilogram in rats.[33] The LD50 of caffeine is dependent on weight and individual sensitivity and estimated to be about 150 to 200 milligrams per kilogram of body mass, roughly 140 to 180 cups of coffee for an average adult taken within a limited timeframe that is dependent on half-life. Though achieving lethal dose with caffeine would be exceptionally difficult with regular coffee, there have been reported deaths from overdosing on caffeine pills.[34][35][36][37]
Treatment of severe caffeine intoxication is generally supportive, providing treatment of the immediate symptoms, but if the patient has very high serum levels of caffeine then peritoneal dialysis, hemodialysis, or hemofiltration may be required.
Anxiety and sleep disorders
Long-term overuse of caffeine can elicit a number of psychiatric disturbances. Two such disorders recognized by the APA are caffeine-induced sleep disorder and caffeine-induced anxiety disorder.
In the case of caffeine-induced sleep disorder, an individual regularly ingests high doses of caffeine sufficient to induce a significant disturbance in his or her sleep, sufficiently severe to warrant clinical attention.[38]
In some individuals, the large amounts of caffeine can induce anxiety severe enough to necessitate clinical attention. This caffeine-induced anxiety disorder can take many forms, from generalized anxiety, to panic attacks, obsessive-compulsive symptoms, or even phobic symptoms.[38] Because this condition can mimic organic mental disorders, such as panic disorder, generalized anxiety disorder, bipolar disorder, or even schizophrenia, a number of medical professionals believe caffeine-intoxicated people are routinely misdiagnosed and unnecessarily medicated when the treatment for caffeine-induced psychosis would simply be to withhold further caffeine.[39] A Study in the British Journal of Addiction concluded that caffeinism, although infrequently diagnosed, may afflict as many as one person in ten of the population.[40]
Pharmacology
Metabolism
Caffeine is completely absorbed by the stomach and small intestine within 45 minutes of ingestion. After ingestion it is distributed throughout all tissues of the body and is eliminated by first-order kinetics.[41]
The half-life of caffeine — the time required for the body to eliminate one-half of the total amount of caffeine consumed at a given time — varies widely among individuals according to such factors as age, liver function, pregnancy, some concurrent medications, and the level of enzymes in the liver needed for caffeine metabolism. In healthy adults, caffeine's half-life is approximately 3-4 hours. In women taking oral contraceptives this is increased to 5-10 hours,[42] and in pregnant women the half-life is roughly 9-11 hours.[43] Caffeine can accumulate in individuals with severe liver disease when its half-life can increase to 96 hours.[44] In infants and young children, the half-life may be longer than in adults; half-life in a newborn baby may be as long as 30 hours. Other factors such as smoking can shorten caffeine's half-life.[45]
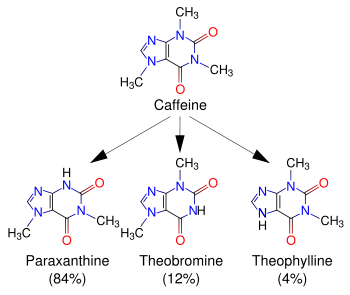
Caffeine is metabolized in the liver by the cytochrome P450 oxidase enzyme system (specifically, the 1A2 isozyme) into three metabolic dimethylxanthines,[46] which each have their own effects on the body:
- Paraxanthine (84%) – Has the effect of increasing lipolysis, leading to elevated glycerol and free fatty acid levels in the blood plasma.
- Theobromine (12%) – Dilates blood vessels and increases urine volume. Theobromine is also the principal alkaloid in cocoa, and therefore chocolate.
- Theophylline (4%) – Relaxes smooth muscles of the bronchi, and is used to treat asthma. The therapeutic dose of theophylline, however, is many times greater than the levels attained from caffeine metabolism.
Each of these metabolites is further metabolized and then excreted in the urine.
Mechanism of action
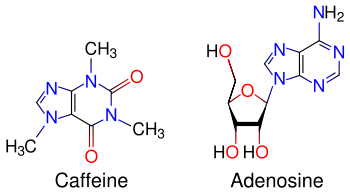
Caffeine acts through multiple mechanisms involving both action on receptors and channels at the cell membrane, as well as intracellular action on Calcium and cAMP pathways. By virtue of its purine structure it can act on some of the same targets as adenosine related nucleosides and nucleotides, like the cell surface P1 GPCRs for adenosine, as well as the intracellular Ryanodine receptor which is the physiological target of cADPR (cyclic ADP ribose), and cAMP-phosphodiesterase (cAMP-PDE). However the action is antagonistic in some cases and agonistic in some others.
The principal mode of action of caffeine is as an antagonist of adenosine receptors in the brain.[47] The caffeine molecule is structurally similar to adenosine, and binds to adenosine receptors on the surface of cells without activating them (a "false transmitter" method of antagonism). The reduction in adenosine activity results in increased activity of the neurotransmitter dopamine, largely accounting for the stimulatory effects of caffeine. Caffeine can also increase levels of epinephrine/adrenaline,[48] possibly via a different mechanism. Acute usage of caffeine also increases levels of serotonin, causing positive changes in mood.
The inhibition of adenosine may be relevant in its diuretic properties. Because adenosine is known to constrict preferentially the afferent arterioles of the glomerulus, its inhibition may cause vasodilation, with an increase in renal blood flow (RBF) and glomerular filtration rate (GFR). This effect, called competitive inhibition, interrupts a pathway that normally serves to regulate nerve conduction by suppressing post-synaptic potentials. The result is an increase in the levels of epinephrine and norepinephrine/noradrenaline released via the hypothalamic-pituitary-adrenal axis.[49] Epinephrine, the natural endocrine response to a perceived threat, stimulates the sympathetic nervous system, leading to an increased heart rate, blood pressure and blood flow to muscles, a decreased blood flow to the skin and inner organs and a release of glucose by the liver.
Caffeine is also a known competitive inhibitor of the enzyme cAMP-phosphodiesterase (cAMP-PDE), which converts cyclic AMP (cAMP) in cells to its noncyclic form, allowing cAMP to build up in cells. Cyclic AMP participates in the messaging cascade produced by cells in response to stimulation by epinephrine, so by blocking its removal caffeine intensifies and prolongs the effects of epinephrine and epinephrine-like drugs such as amphetamine, methamphetamine, or methylphenidate. Increased concentrations of cAMP in parietal cells causes an increased activation of protein kinase A (PKA) which in turn increases activation of H+/K+ ATPase, resulting finally in increased gastric acid secretion by the cell.
Caffeine (and theophylline) can freely diffuse into cells and causes intracellular calcium release (independent of extracellular calcium) from the calcium stores in the Endoplasmic Reticulum(ER). This release is only partially blocked by Ryanodine receptor blockade with ryanodine, dantrolene, ruthenium red, and procaine (thus may involve ryanodine receptor and probably some additional calcium channels), but completely abolished after calcium depletion of ER by SERCA inhibitors like Thapsigargin (TG) or cyclopiazonic acid (CPA).[50] The action of caffeine on the ryanodine receptor may depend on both cytosolic and the luminal ER concentrations of Ca2+. At low millimolar concentration of caffeine, the RyR channel open probability (Po) is significantly increased mostly due to a shortening of the lifetime of the closed state. At concentrations >5 mM, caffeine opens RyRs even at picomolar cytosolic Ca2+ and dramatically increases the open time of the channel so that the calcium release is stronger than even an action potential can generate. This mode of action of caffeine is probably due to mimicking the action of the physiologic metabolite of NAD called cADPR (cyclic ADP ribose) which has a similar potentiating action on Ryanodine receptors.
Caffeine may also directly inhibit delayed rectifier and A-type K+ currents and activate plasmalemmal Ca2+ influx in certain vertebrate and invertebrate neurons.
The metabolites of caffeine contribute to caffeine's effects. Theobromine is a vasodilator that increases the amount of oxygen and nutrient flow to the brain and muscles. Theophylline, the second of the three primary metabolites, acts as a smooth muscle relaxant that chiefly affects bronchioles and acts as a chronotrope and inotrope that increases heart rate and efficiency. The third metabolic derivative, paraxanthine, is responsible for an increase in the lipolysis process, which releases glycerol and fatty acids into the blood to be used as a source of fuel by the muscles.[51]
Tolerance and withdrawal
Because caffeine is primarily an antagonist of the central nervous system's receptors for the neurotransmitter adenosine, the bodies of individuals who regularly consume caffeine adapt to the continual presence of the drug by substantially increasing the number of adenosine receptors in the central nervous system. This increase in the number of the adenosine receptors makes the body much more sensitive to adenosine, with two primary consequences.[52] First, the stimulatory effects of caffeine are substantially reduced, a phenomenon known as a tolerance adaptation. Second, because these adaptive responses to caffeine make individuals much more sensitive to adenosine, a reduction in caffeine intake will effectively increase the normal physiological effects of adenosine, resulting in unwelcome withdrawal symptoms in tolerant users.[52]
Because adenosine, in part, serves to regulate blood pressure by causing vasodilation, the increased effects of adenosine cause the blood vessels of the head to dilate, leading to an excess of blood in the head and causing a headache and nausea. Reduced catecholamine activity may cause feelings of fatigue and drowsiness. A reduction in serotonin levels when caffeine use is stopped can cause anxiety, irritability, inability to concentrate and diminished motivation to initiate or to complete daily tasks; in extreme cases it may cause mild depression.
Withdrawal symptoms — possibly including headache, irritability, and an inability to concentrate — may appear within 12 to 24 hours after discontinuation of caffeine intake, peak at roughly 48 hours, and usually last from one to five days - representing the time required for the number of adenosine receptors in the brain to revert to "normal" levels, uninfluenced by caffeine consumption. Analgesics, such as aspirin, can relieve the pain symptoms, as can a small dose of caffeine.[53] Most effective is a combination of both an analgesic and a small amount of caffeine.
Currently caffeine withdrawal is recognized as meriting further study by the Diagnostic and Statistical Manual of Mental Disorders for DSM-IV, although research demonstrating its clinical significance means that it will likely be included as an Axis-1 disorder in the DSM-V.[54]
Extraction of pure caffeine

Caffeine extraction is an important industrial process and can be performed using a number of different solvents. Benzene, chloroform, trichloroethylene and dichloromethane have all been used over the years but for reasons of safety, environmental impact, cost and flavor, they have been superseded by the following main methods:
Water extraction
Coffee beans are soaked in water. The water, which contains not only caffeine but also many other compounds which contribute to the flavor of coffee, is then passed through activated charcoal, which removes the caffeine. The water can then be put back with the beans and evaporated dry, leaving decaffeinated coffee with a good flavor.[55] Coffee manufacturers recover the caffeine and resell it for use in soft drinks and medicines.
Supercritical carbon dioxide extraction
Supercritical carbon dioxide is an excellent nonpolar solvent for caffeine (as well as many other organic compounds), and is safer than the organic solvents that are used for caffeine extraction. The extraction process is simple: CO2 is forced through the green coffee beans at temperatures above 31.1 °C and pressures above 73 atm. Under these conditions, CO2 is in a "supercritical" state: it has gaslike properties which allow it to penetrate deep into the beans but also liquid-like properties which dissolve 97-99% of the caffeine. The caffeine-laden CO2 is then sprayed with high pressure water to remove the caffeine. The caffeine can then be isolated by charcoal adsorption (as above) or by distillation, recrystallization, or reverse osmosis.[55]
Extraction by nonhazardous organic solvents
Organic solvents such as ethyl acetate present much less health and environmental hazard than previously used chlorinated and aromatic solvents. The hydrolysis products of ethyl acetate are ethanol and acetic acid, both nonhazardous in small quantities. Another method is to use triglyceride oils obtained from spent coffee grounds.
References
- ^ Lovett, Richard (24 September 2005). "Coffee: The demon drink?". New Scientist (2518).
- ^ Caffeine Content of Food and Drugs. Nutrition Action Health Newsletter. Center For Science in the Public Interest (December 1996). Retrieved on 2006-08-22.
- ^ Erowid (July 7, 2006). Caffeine Content of Beverages, Foods, & Medications. The Vaults of Erowid. Retrieved on 2006-08-22.
- ^ Nathanson, JA (12 October 1984). "Caffeine and related methylxanthines: possible naturally occurring pesticides". Science 226 (4671): 184-7. PMID 6207592.
- ^ Erowid (Dec 2003). Does Yerba Maté Contain Caffeine or Mateine?. The Vaults of Erowid. Retrieved on 2006-08-16.
- ^ PubChem: mateina. National Library of Medicine. Retrieved on 2006-08-16.. Generally translated as mateine in articles written in English
- ^ PubChem: guaranine. National Library of Medicine. Retrieved on 2006-08-16.
- ^ a b Caffeine. International Coffee Organization. Retrieved on 2006-08-21.
- ^ Caffeine in tea vs. steeping time (September 1996). Retrieved on 2006-08-12.
- ^ Smit, HJ, Gaffan EA, Rogers PJ. (2004 Nov). "Methylxanthines are the psycho-pharmacologically active constituents of chocolate". Psychopharmacology 176 (3-4): 412-9.
- ^ Haskell, CF, Kennedy D, Wesnes KA, Milne AL, Scholey AB (13 March 2006). "A double-blind, placebo-controlled, multi-dose evaluation of the acute behavioural effects of guarana in humans". J Psychopharmacol 0 (0): 0-0. PMID 16533867.; [Epub ahead of print]
- ^ Escohotado, Antonio, Ken Symington (May 1999). A Brief History of Drugs: From the Stone Age to the Stoned Age. Park Street Press. ISBN 0-89281-826-3.
- ^ Chow p. 19-20 (Czech edition); also Arcimovicova p. 9, Evans p. 2 and others
- ^ Yu, Lu (October 1995). The Classic of Tea: Origins & Rituals. Ecco Pr; Reissue edition. ISBN 0-88001-416-4.
- ^ "Coffee". Encyclopædia Britannica. (1911).
- ^ Benjamin, LT Jr, Rogers AM, Rosenbaum A (1991 Jan). "Coca-Cola, caffeine, and mental deficiency: Harry Hollingworth and the Chattanooga trial of 1911". J Hist Behav Sci 27 (1): 42-55. PMID 2010614.
- ^ Jarvis, Gail (May 21, 2002). The Rise and Fall of Cocaine Cola. Retrieved on 2006-08-19.
- ^ Weinberg, BA, BK Bealer (January 2001). The World of Caffeine. Routledge. ISBN 0-415-92722-6.
- ^ Whats your poison: caffeine. Australian Broadcasting Corporation (1997). Retrieved on 2006-08-20.
- ^ Nehlig, A, Daval JL, Debry G (1992 May-Aug). "Caffeine and the central nervous system: Mechanisms of action, biochemical, metabolic, and psychostimulant effects". Brain Res Brain Res Rev 17 (2): 139-70. PMID 1356551.
- ^ a b Bolton, Ph.D., Sanford, Gary Null, M.S. (1981). "Caffeine: Psychological Effects, Use and Abuse". Orthomolecular Psychiatry 10 (3): 202-211. Retrieved on 2006-08-12.
- ^ Ivy, JL, Costill DL, Fink WJ, Lower RW (1979 Spring). "Influence of caffeine and carbohydrate feedings on endurance performance". Med Sci Sports 11 (1): 6-11. PMID 481158.
- ^ Graham, TE, Spriet, LL (1991 Dec). "Performance and metabolic responses to a high caffeine dose during prolonged exercise". J Appl Physiol 71 (6): 2292-8. PMID 1778925.
- ^ Trice, I, Haymes, EM (Mar 1995). "Effects of caffeine ingestion on exercise-induced changes during high-intensity, intermittent exercise". Int J Sport Nutr 5 (1): 37-44. PMID 7749424.
- ^ Headache Triggers: Caffeine. WebMD (June 2004). Retrieved on 2006-08-14.
- ^ Caffeine (Systemic). MedlinePlus (05/25/2000). Retrieved on 2006-08-12.
- ^ Schmidt, B, Roberts, RS, Davis, P, Doyle, LW, et al (May 18, 2006). "Caffeine therapy for apnea of prematurity". N Engl J Med 354 (20): 2112-21.
- ^ Noever, R., J. Cronise, and R. A. Relwani. 1995. Using spider-web patterns to determine toxicity. NASA Tech Briefs 19(4):82. Published in New Scientist magazine, 27 April 1995.
- ^ a b Caffeine-related disorders. Encyclopedia of Mental Disorders. Retrieved on 2006-08-14.
- ^ Gastroesophageal Reflux Disease (GERD). Cedars-Sinai. Retrieved on 2006-08-14.
- ^ Erowid Caffeine Vault: Effects. The Vaults of Erowid (Jul 08, 2006). Retrieved on 2006-08-14.
- ^ Kamijo, Y, Soma K, Asari Y, Ohwada T (1999 Dec). "Severe rhabdomyolysis following massive ingestion of oolong tea: caffeine intoxication with coexisting hyponatremia". Veterinary and Human Toxicology 41 (6): 381-3. PMID 10592946.
- ^ Erowid Caffeine Vault: Caffeine Dosage. The Vaults of Erowid (Jul 08, 2006). Retrieved on 2006-08-14.
- ^ Kerrigan, S, Lindsey T (2005). "Fatal caffeine overdose: two case reports". Forensic Sci Int 153 (1): 67-9.
- ^ Holmgren, P, Norden-Pettersson L, Ahlner J (2004). "Caffeine fatalities — four case reports". Forensic Sci Int 139 (1): 71-3.
- ^ Walsh, I, Wasserman GS, Mestad P, Lanman RC (Dec 1987). "Near-fatal caffeine intoxication treated with peritoneal dialysis". Pediatr Emerg Care 3 (4): 244-9. PMID 3324064.
- ^ Mrvos, RM, Reilly PE, Dean BS, Krenzelok EP (Dec 1989). "Massive caffeine ingestion resulting in death". Vet Hum Toxicol 31 (6): 571-2. PMID 2617841.
- ^ a b (1994) Diagnostic and Statistical Manual of Mental Disorders, fourth Edition.. American Psychiatric Association. ISBN 0-89042-062-9.
- ^ Shannon, MW, Haddad LM, Winchester JF (1998). Clinical Management of Poisoning and Drug Overdose, 3rd ed.. ISBN 0-7216-6409-1.
- ^ James, JE, KP Stirling (Sep 1983). "Caffeine: A summary of some of the known and suspected deleterious effects of habitual use". British Journal of Addiction 78 (3): 251-8. PMID 6354232.
- ^ Newton, R, Broughton LJ, Lind MJ, Morrison PJ, Rogers HJ, Bradbrook ID (1981). "Plasma and salivary pharmacokinetics of caffeine in man". European Journal of Clinical Pharmacology 21 (1): 45-52. PMID 7333346.
- ^ Meyer, FP, Canzler E, Giers H, Walther H. (1991). "Time course of inhibition of caffeine elimination in response to the oral depot contraceptive agent Deposiston. Hormonal contraceptives and caffeine elimination". Zentralbl Gynakol 113 (6): 297-302. PMID 2058339.
- ^ Ortweiler, W, Simon HU, Splinter FK, Peiker G, Siegert C, Traeger A. (1985). "Determination of caffeine and metamizole elimination in pregnancy and after delivery as an in vivo method for characterization of various cytochrome p-450 dependent biotransformation reactions". Biomed Biochim Acta. 44 (7-8): 1189-99. PMID 4084271.
- ^ Bolton, Ph.D., Sanford, Gary Null, M.S. (1981). "Caffeine: Psychological Effects, Use and Abuse". Orthomolecular Psychiatry 10 (3): 202-211. Retrieved on 2006-08-14.
- ^ Springhouse (January 1, 2005). Physician's Drug Handbook; 11th edition. Lippincott Williams & Wilkins. ISBN 1-58255-396-3.
- ^ Caffeine. The Pharmacogenetics and Pharmacogenomics Knowledge Base. Retrieved on 2006-08-14.
- ^ Fisone G, G, Borgkvist A, Usiello A (2004 Apr). "Caffeine as a psychomotor stimulant: mechanism of action". Cell Mol Life Sci 61 (7-8): 857-72. PMID 15095008.
- ^ Graham T, Rush J, van Soeren M (1994). "Caffeine and exercise: metabolism and performance.". Can J Appl Physiol 19 (2): 111-38. PMID 8081318.
- ^ Fredholm B, Bättig K, Holmén J, Nehlig A, Zvartau E (1999). "Actions of caffeine in the brain with special reference to factors that contribute to its widespread use.". Pharmacol Rev 51 (1): 83-133. PMID 10049999.Full text
- ^ Alexei Verkhratsky Physiology and Pathophysiology of the Calcium Store in the Endoplasmic Reticulum of Neurons Physiol. Rev. 85: 201-279, 2005. doi:10.1152/physrev.00004.2004
- ^ Dews, P.B. (1984). "Caffeine: Perspectives from Recent Research". Berlin: Springer-Valerag
- ^ a b Green, RM, Stiles GL (Jan 1986). "Chronic caffeine ingestion sensitizes the A1 adenosine receptor-adenylate cyclase system in rat cerebral cortex". J Clin Invest 77 (1): 222-227. PMID 3003150.
- ^ Sawynok, J (Jan 1995). "Pharmacological rationale for the clinical use of caffeine.". Drugs 49 (1): 37-50. PMID 7705215. Retrieved on 2006-08-14.
- ^ Kirchheimer, Sid. "Caffeine Withdrawal Is Real", CBS News, 30 September 2004. Retrieved on 2006-08-14.
- ^ a b Senese, Fred (2005-09-20). How is coffee decaffeinated?. General Chemistry Online. Retrieved on 2006-08-21.
External links
- Caffeine: How Stuff Works
- National Geographic January 2005
- Erowid Caffeine Vaults
- Caffeine Information Archive
- US National Library of Medicine: MedlinePlus Drug Information: Caffeine
- Naked Scientists Online: Why do plants make caffeine?
- Is Caffeine a Health Hazard?
- The Coffee and Caffeine FAQ
- The Physician and Sportsmedicine: Caffeine: A User's Guide
- Caffeine: The Inside Scoop
- Caffeine: Psychological Effects, Use & Abuse
- Caffeine 3D view and pdb-file
- Alcohol and Drugs History Society: Caffeine news page
- eMedicine Caffeine-Related Psychiatric Disorders
- The Consumers Union Report on Licit and Illicit Drugs, Caffeine-Part 1 Part 2
- Coffee: A Little Really Does Go a Long Way, NPR, September 28, 2006
- NASA’s Caffeine Findings (the web of a caffeinated spider)
- Caffeine in the news - news archives
Appendix
Relative content: comparison of different sources
| Caffeine equivalents |
|---|
In general, each of the following contains
approximately 200 milligrams
of caffeine:
|




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xxx
216.73.xxx.xxx
 Server Time:
Server Time: