Contents |
History
Bisphosphonates were developed in the 19th century, but were first investigated in the 1960s for use in disorders of bone metabolism. Their non-medical use included water softening in irrigation systems used in orange groves. The initial rationale for their use in humans was their potential in preventing the dissolution of hydroxylapatite, the principal bone mineral, and hence arresting bone loss. Only in the 1990s was their actual mechanism of action demonstrated.[1]
Chemistry and classes
All bisphosphonate drugs share a common P-C-P "backbone":
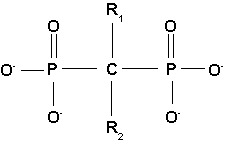
The two PO3 (phosphate) groups covalently linked to carbon determine both the name "bisphosphonate" and the function of the drugs.
The long side chain (R2 in the diagram) determines the chemical properties, the mode of action and the strength of bisphosphonate drugs. The short side chain (R1) mainly influences chemical properties and pharmacokinetics.
Pharmacokinetics
Of the bisphosphonate that is resorbed (from oral preparation) or infused (for intravenous drugs), about 50% is excreted unchanged by the kidney. The remainder has a very high affinity for bone tissue, and is rapidly absorbed onto the bone surface.
Mechanism of action
Bisphosphonates, when attached to bone tissue, are "ingested" by osteoclasts, the bone cell that breaks down bone tissue. The two types of bisphonates work differently in killing osteoclast cells.
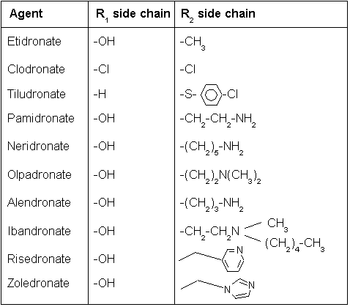
There are two classes of bisphosphonate: the N-containing and non-N-containing bisphosphonates.
Non-nitrogenous
Non-N-containing bisphosphonates:
- Etidronate (Didronel®) - 1 (potency relative to that of etidronate)
- Clodronate (Bonefos®, Loron®) - 10
- Tiludronate (Skelid®) - 10
The non-nitrogenous bisphosphonates are metabolised in the cell to compounds that compete with adenosine triphosphate (ATP) in the cellular energy metabolism. The osteoclast initiates apoptosis and dies, leading to an overall decrease in the breakdown of bone.[2]
Nitrogenous
N-containing bisphosphonates:
- Pamidronate (APD, Aredia®) - 100
Neridronate - 100
Olpadronate - 500
Alendronate (Fosamax®) - 500
Ibandronate (Bondronat®) - 1000
Risedronate (Actonel®) - 2000
Zoledronate (Zometa®) - 10000
Nitrogenous bisphosphonates act on bone metabolism by binding and blocking the enzyme farnesyl diphosphate synthase (FPPS) in the HMG-CoA reductase pathway (also known as the mevalonate pathway).[3]
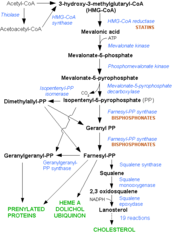
Disruption of the HMG CoA-reductase pathway at the level of FPPS prevents the formation of two metabolites (farnesol and geranylgeraniol) that are essential for connecting some small proteins to the cell membrane. This phenomenon is known as prenylation, and is important for proper sub-cellular protein trafficking (see "lipid anchored protein" for the principles of this phenomenon).[4]
While inhibition of protein prenylation may affect many proteins found in an osteoclast, disruption to the lipid modifcation of Ras, Rho, Rac proteins has been speculated to underlie the effects of bisphosphonates. These proteins can affect both osteoclastogenesis, cell survival, and cytoskeletal dynamics. In particular, the cytoskeleton is vital for maintaining the "ruffled border" that is required for contact between a resorbing osteoclast and a bone surface.
Statins are another class of drugs that inhibit the HMG-CoA reductase pathway. Unlike bisphosphonates, statins do not bind to bone surfaces with high affinity, and are thus not specific for bone. Nevertheless, some studies have reported a decreased rate of fracture (an indicator of osteoporosis) and/or an increased bone mineral density in statin users. The overall efficacy of statins in the treatment osteoporosis remains controversial.
Uses
Bisphosphonates are used clinically for the treatment of osteoporosis, osteitis deformans (Paget's disease of the bone), bone metastasis (with or without hypercalcemia), multiple myeloma and other conditions that feature bone fragility.
In osteoporosis and Paget's, alendronate and risedronate are the most popular first-line drugs. If these are ineffective or the patient develops digestive tract problems, intravenous pamidronate may be used. Alternatively, strontium ranelate or teriparatide are used for refractory disease, and the SERM raloxifene is occasionally administered in postmenopausal women instead of bisphosphonates.
High-potency intravenous bisphosphonates have shown to modify progression of skeletal metastasis in several forms of cancer, especially breast cancer.
More recently, bisphosphonates have been used to reduce fracture rates in children with osteogenesis imperfecta.
Side-effects
- Oral bisphosphonates can give stomach upset and inflammation and erosions of the esophagus, which is the main problem of oral N-containing preparations. This can be prevented by remaining seated upright for 30 to 60 minutes after taking the medication.
- Intravenous bisphosphonates can give fever and flu-like symptoms after the first infusion. Notably, these symptoms do not recur with subsequent infusions.
- There is a slightly increased risk for electrolyte disturbances, but not enough to warrant regular monitoring.
- In chronic renal failure, the drugs are excreted much slower, and dose adjustment is required.
- Bisphosphonates have been associated with osteonecrosis of the jaw; with the mandible twice as frequently affected as the maxilla and most cases occurring following high-dose intravenous administration used for some cancer patients. Some 60% of cases are preceded by a dental surgical procedure and it has been suggested that bisphosphonate treatment should be postponed until after any dental work to eliminate potential sites of infection.[5]
- A number of cases of severe bone, joint or musculoskeletal pain have been reported, prompting labeling changes[6]
Footnotes
- ^ Fleisch H (2002). "Development of bisphosphonates.". Breast Cancer Res 4 (1): 30-4. PMID 11879557.
- ^ Frith J, Mönkkönen J, Blackburn G, Russell R, Rogers M (1997). "Clodronate and liposome-encapsulated clodronate are metabolized to a toxic ATP analog, adenosine 5'-(beta, gamma-dichloromethylene) triphosphate, by mammalian cells in vitro.". J Bone Miner Res 12 (9): 1358-67. PMID 9286751.
- ^ van Beek E, Cohen L, Leroy I, Ebetino F, Löwik C, Papapoulos S (Nov 2003). "Differentiating the mechanisms of antiresorptive action of nitrogen containing bisphosphonates.". Bone 33 (5): 805-11. PMID 14623056.
- ^ van beek E, Löwik C, van der Pluijm G, Papapoulos S (1999). "The role of geranylgeranylation in bone resorption and its suppression by bisphosphonates in fetal bone explants in vitro: A clue to the mechanism of action of nitrogen-containing bisphosphonates.". J Bone Miner Res 14 (5): 722-9. PMID 10320520.
- ^ Woo S, Hellstein J, Kalmar J (2006). "Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws.". Ann Intern Med 144 (10): 753-61. PMID 16702591.
- ^ Wysowski D, Chang J (2005). "Alendronate and risedronate: reports of severe bone, joint, and muscle pain.". Arch Intern Med 165 (3): 346-7. PMID 15710802.




 216.73.216.103
216.73.216.103 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xxx
216.73.xxx.xxx
 Server Time:
Server Time: