| Acetone | |
|---|---|
  |
|
| General | |
| Systematic name | Propanone |
| Other names | β-ketopropane Dimethyl ketone |
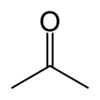
| Molecular formula | CH3COCH3 |
| SMILES | CC(=O)C |
| Molar mass | 58.09 g/mol |
| Appearance | Colorless liquid |
| CAS number | [67-64-1] |
| Properties | |
| Density and phase | 0.79 g/cm³, liquid |
| Solubility in water | miscible |
| Melting point | −94.9 °C (178.2 K) |
| Boiling point | 56.3 °C (329.4 K) |
| Viscosity | 0.32 cP at 20 °C |
| Structure | |
| Molecular shape | trigonal planar at C=O |
| Dipole moment | 2.91 D |
| Hazards | |
| MSDS | External MSDS |
| EU classification | Flammable (F) Irritant (Xi) |
| NFPA 704 |

3
1
0
|
| R-phrases | R11, R36, R66, R67 |
| S-phrases | S2, S9, S16, S26 |
| Flash point | −20 °C |
| Autoignition temperature | 465 °C |
| RTECS number | AL31500000 |
| Supplementary data page | |
| Structure & properties | n, εr, etc. |
| Thermodynamic data | Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Related ketones | Butanone |
| Related solvents |
water Ethanol Isopropanol Toluene |
|
Except where noted otherwise, data are given
for materials in their standard state (at 25°C, 100 kPa) |
|
In chemistry, acetone (also known as propanone, dimethyl ketone, 2-propanone, propan-2-one and β-ketopropane) is the simplest representative of the ketones.
Acetone is a colorless mobile flammable liquid with melting point at −95.4 °C and boiling point at 56.53 °C. It has a relative density of 0.819 (at 0 °C). It is readily soluble in water, ethanol, ether, etc., and itself serves as an important solvent. The most familiar household use of acetone is as the active ingredient in nail polish remover. Acetone is also used to make plastic, fibers, drugs, and other chemicals.
Contents |
Uses
An important industrial use for acetone involves its reaction with phenol for the manufacture of bisphenol A. Bisphenol A is an important component of many polymers such as polycarbonates, polyurethanes and epoxy resins. Acetone is also used extensively for the safe transporting and storing of acetylene. Vessels containing a porous material are first filled with acetone followed by acetylene, which dissolves into the acetone. One liter of acetone can dissolve around 250 litres of acetylene.
Acetone is often the primary (or only) component in nail polish remover. Acetone is also used as a superglue remover. It can be used for thinning and cleaning fiberglass resins and epoxies. It is a strong solvent for most plastics and synthetic fibres.
Acetone has been used in the manufacture of cordite. During World War I a new process of producing acetone through bacterial fermentation was developed by Chaim Weizmann, to help the British war effort.
Acetone can also dissolve many plastics, including those used in consumer-targeted Nalgene bottles.
Acetone is also used as a drying agent, due to the readiness with which it binds to water, and its volatility.
Acetone can also be used on the hair. It can be used as a rinse before shampooing to remove build up, oil and hard water minerals.
Uses in universities and professional laboratories
Due to the nonpolar properties of acetone, it has been used in many university labs as a cleanser for nonpolar substances in testtubes after working with nonpolar organic substances (i.e. cyclohexane, cyclohexene). Acetone is squirted directly from a bottle containing the substance onto the glassware and is drained into a specially marked organic waste bucket.
However, care should be used when cleaning testtubes with acetone as acetone is readily absorbed through vinyl or latex gloves and will come in contact with skin where it is absorbed into the skin. If an excessive amount of acetone is splashed onto gloves, remove gloves immediately and wash hands with water before donning a new pair of gloves.
Acetone is very cool to the touch due to its high volatility and will evaporate quickly, making it very useful in drying lab glassware. Avoid inhaling fumes as acetone is an irritant and inhalation may lead to hepatanic effects (causing liver damage). Always use goggles when handling acetone, it can cause permanent eye damage (corneal clouding).
Health effects
Small amounts of acetone are metabolically produced in the body, mainly from fat. In humans, fasting significantly increases its endogenous production (see ketosis). Acetone can be elevated in diabetes. Exposure to exogenous acetone can be chronic due to acetone contamination of water, food (e.g. milk), or the air (acetone is volatile). A number of acute poisoning cases have been described. Relatively speaking, acetone is not a very toxic compound; it can, however, damage the mucosa of the mouth and can irritate and damage skin. Accidental intake of large amounts of acetone may lead to unconsciousness and death.
The effects of long-term exposure to acetone are known mostly from animal studies. Kidney, liver, and nerve damage, increased birth defects, and lowered reproduction ability of males (only) occurred in animals exposed long-term. It is not known if these same effects would be exhibited in humans.
Interestingly, acetone has been shown to have anticonvulsant effects in animal models of epilepsy, in the absence of toxicity, when administered in millimolar concentrations (see: Likhodii et. al., Ann Neurol. 2003;54(2):219-226). It has been hypothesized that the high fat low carbohydrate ketogenic diet used clinically to control drug-resistant epilepsy in children works by elevating acetone in the brain (see Ibid, p. 219).
External links
- International Chemical Safety Card 0087
- National Pollutant Inventory – Acetone
- NIOSH Pocket Guide to Chemical Hazards
- Link page to external chemical sources.




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xxx
216.73.xxx.xxx
 Server Time:
Server Time: