 |
|
|
Vancomycin
|
|
| Systematic (IUPAC) name | |
| unable to be assigned | |
| Identifiers | |
| CAS number | 1404-90-6 |
| ATC code | A07AA09 J01XA01 |
| PubChem | 14969 |
| DrugBank | APRD01287 |
| Chemical data | |
| Formula | C66H75N9Cl2O24 |
| Mol. weight | 1449.3 g.mol-1 |
| Pharmacokinetic data | |
| Bioavailability | Negligible (oral) |
| Metabolism | Excreted unchanged |
| Half life | 411 hours (adults) 6-10 days (adults, impaired renal function) |
| Excretion | Renal |
| Therapeutic considerations | |
| Pregnancy cat. | B2 (Au), B (U.S.) |
| Legal status | S4 (Au), POM (UK), ℞-only (U.S.) |
| Routes | IV, oral |
Vancomycin (INN) (IPA: [ˌvæŋkoˈmaɪsən]) is a glycopeptide antibiotic used in the prophylaxis and treatment of infections caused by Gram-positive bacteria. It has traditionally been reserved as a drug of "last resort", used only after treatment with other antibiotics had failed, although the emergence of vancomycin-resistant organisms means that it is increasingly being displaced from this role by linezolid and the carbapenems.
It was developed by Eli Lilly, which marketed vancomycin hydrochloride under the trade name Vancocin. In 2004, Eli Lilly licensed Vancocin to ViroPharma in the U.S., Flynn Pharma in the UK and Aspen Pharmacare in Australia. The patent expired in the early 1980s and generic versions of the drug are also available under various trade names.
Contents |
Pharmacology and chemistry
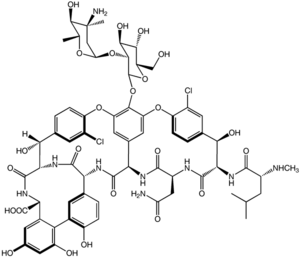
It is a branched tricyclic glycosylated nonribosomal peptide produced by the fermentation of the Actinobacteria species Amycolatopsis orientalis (formerly designated Nocardia orientalis).
Vancomycin acts by inhibiting proper cell wall synthesis in Gram-positive bacteria. The mechanism inhibited, and various factors related to entering the outer membrane of Gram-negative organisms mean that vancomycin is not active against Gram-negative bacteria (except some non-gonococcal species of Neisseria).
Specifically, vancomycin prevents incorporation of N-acetylmuramic acid (NAM)- and N-acetylglucosamine (NAG)-peptide subunits into the peptidoglycan matrix; which forms the major structural component of Gram-positive cell walls.
The large hydrophilic molecule is able to form hydrogen bond interactions with the terminal D-alanyl-D-alanine moieties of the NAM/NAG-peptides. Normally this is a five-point interaction. This binding of vancomycin to the D-Ala-D-Ala prevents the incorporation of the NAM/NAG-peptide subunits into the peptidoglycan matrix.
Vancomycin exhibits atropisomerism it has two chemically distinct rotamers owing to the rotational restriction of the chlorotyrosine residue (on the right hand side of the figure). The form present in the drug is the thermodynamically more stable conformer, and, importantly, has more potent activity.
Clinical use
Indications
Vancomycin is indicated for the treatment of serious, life-threatening infections by Gram-positive bacteria which are unresponsive to other less toxic antibiotics.
The increasing emergence of vancomycin-resistant enterococci has resulted in the development of guidelines for use by the Centers for Disease Control (CDC) Hospital Infection Control Practices Advisory Committee. These guidelines restrict use of vancomycin to the following indications:[1]
- treatment of serious infections caused by susceptible organisms resistant to penicillins (methicillin-resistant Staphylococcus aureus and multi-resistant Staphylococcus epidermidis (MRSE)) or in individuals with serious allergy to penicillins
- pseudomembranous colitis (relapse or unresponsive to metronidazole treatment)
- For treatment of infections caused by gram-positive microorganisms in patients who have serious allergies to beta-lactam antimicrobials. (http://wonder.cdc.gov/wonder/prevguid/m0039349/m0039349.asp)
- antibacterial prophylaxis for endocarditis following certain procedures in penicillin-hypersensitive individuals at high risk
- surgical prophylaxis for major procedures involving implantation of prostheses in institutions with a high rate of MRSA or MRSE
Adverse effects
Common adverse drug reactions (≥1% of patients) associated with IV vancomycin include: local pain, which may be severe and/or thrombophlebitis. Nephrotoxicity is an infrequent adverse effect (0.11% of patients). Rare adverse effects (<0.1% of patients) include: anaphylaxis, toxic epidermal necrolysis, erythema multiforme, red man syndrome (see below), superinfection, thrombocytopenia, neutropenia, leucopenia, tinnitus, dizziness and/or ototoxicity (see below).[1]
Dosing considerations
Intravenous vs oral administration
Vancomycin needs to be given intravenously (IV) for systemic therapy since it does not cross through the intestinal lining. It is a large hydrophilic molecule which partitions poorly across the gastrointestinal mucosa. The only indication for oral vancomycin therapy is in the treatment of pseudomembranous colitis, where it must be given orally to reach the site of infection in the colon.
Red man syndrome
Vancomycin must be administered in a dilute solution slowly, over at least 60 minutes (maximum rate of 10 mg/minute for doses >500 mg).[1] This is due to the high incidence of pain and thrombophlebitis and to avoid an infusion reaction known as the red man syndrome or red neck syndrome. This syndrome, usually appearing within 410 minutes after the commencement or soon after the completion of an infusion, is characterised by flushing and/or and an erythematous rash that affects the face, neck and upper torso. Less frequently, hypotension and angioedema may also occur. Symptoms may be treated with antihistamines, including diphenhydramine.[2]
Therapeutic drug monitoring
Vancomycin activity is considered to be time-dependent that is, antimicrobial activity depends on the duration that the drug level exceeds the minimum inhibitory concentration (MIC) of the target organism. Thus, peak levels have not been shown to correlate with efficacy or toxicity indeed concentration monitoring is unnecessary in most cases. Circumstances where therapeutic drug monitoring (TDM) is warranted include: patients receiving concomitant aminoglycoside therapy, patients with (potentially) altered pharmacokinetic parameters, patients on haemodialysis, during high dose or prolonged treatment, and patients with impaired renal function. In such cases, trough concentrations are measured.[1][3][4][5]
Toxicity
Vancomycin has traditionally been considered a nephrotoxic and ototoxic drug, based on observations by early investigators of elevated serum levels in renally impaired patients who had experienced ototoxicity, and subsequently through case reports in the medical literature. However, as the use of vancomycin increased with the spread of MRSA beginning in the seventies, it was recognised that the previously reported rates of toxicity were not being observed. This was attributed to the removal of the impurities present in the earlier formulation of the drug, although those impurities were not specifically tested for toxicity.
Nephrotoxicity
Subsequent reviews of accumulated case reports of vancomycin-related nephrotoxicity found that many of the patients had also received other known nephrotoxins, particularly aminoglycosides. Most of the rest had other confounding factors, or insufficient data regarding the possibility of such, that prohibited the clear association of vancomycin with the observed renal dysfunction.
In 1994, Cantu and colleagues found that the use of vancomycin monotherapy was clearly documented in only three of 82 available cases in the literature.[3] Prospective and retrospective studies attempting to evaluate the incidence of vancomycin-related nephrotoxicity have largely been methodologically flawed and have produced variable results. The most methodologically sound investigations indicate that the actual incidence of vancomycin-induced nephrotoxicity is around 57%. To put this into context, similar rates of renal dysfunction have been reported for cefamandole and benzylpenicillin, two reputedly non-nephrotoxic antibiotics.
Additionally, evidence to relate nephrotoxicity to vancomycin serum levels is inconsistent. Some studies have indicated an increased rate of nephrotoxicity when trough levels exceed 10 µg/mL, but others have not reproduced these results. Nephrotoxicity has also been observed with concentrations within the "therapeutic" range as well. Essentially, the reputation of vancomycin as a nephrotoxin is over-stated, and it has not been demonstrated that maintaining vancomycin serum levels within certain ranges will prevent its nephrotoxic effects, when they do occur.
Ototoxicity
Attempts to establish rates of vancomycin-induced ototoxicity are even more difficult due to the scarcity of quality evidence. The current consensus is that clearly related cases of vancomycin ototoxicity are rare. The association between vancomycin serum levels and ototoxicity is also uncertain. While cases of ototoxicity have been reported in patients whose vancomycin serum level exceeded 80 µg/mL, cases have been reported in patients with therapeutic levels as well. Thus, it also remains unproven that therapeutic drug monitoring of vancomycin for the purpose of maintaining "therapeutic" levels will prevent ototoxicity.
Interactions with other nephrotoxins
Another area of controversy and uncertainty concerns the question of whether, and if so, to what extent, vancomycin increases the toxicity of other nephrotoxins. Clinical studies have yielded variable results, but animal models indicate that there probably is some increased nephrotoxic effect when vancomycin is added to nephrotoxins such as aminoglycosides. However, a dose- or serum level-effect relationship has not been established.
Antibiotic resistance
Microbial resistance to vancomycin is a growing problem, particularly within health care facilities such as hospitals. With vancomycin being the last-line antibiotic for serious Gram-positive infections there is the growing prospect that resistance will result in a return to the days when fatal bacterial infections were common. Vancomycin-resistant enterococci (VRE) emerged in 1987. Vancomycin-resistance emerged in more common pathogenic organisms during the 1990s and 2000s, including vancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-resistant Staphylococcus aureus (VRSA), and vancomycin-resistant Clostridium difficile.[6][7] There is some suspicion that agricultural use of avoparcin, another similar glycopeptide antibiotic, has contributed to the emergence of vancomycin-resistant organisms.
One mechanism of resistance to vancomycin appears to be alteration to the terminal amino acid residues of the NAM/NAG-peptide subunits, normally D-alanyl-D-alanine, which vancomycin binds to. Variations such as D-alanyl-D-lactate and D-alanyl-D-serine result in only a 4-point hydrogen bonding interaction being possible between vancomycin and the peptide. This loss of just one point of interaction results in a 1000-fold decrease in affinity.
In Enterococci this modification appears to be due to the expression of an enzyme which alters the terminal residue. Three main resistance variants have been characterised to date among resistant Enterococcus faecium and E. faecalis populations.
- VanA - resistance to vancomycin and teicoplanin, inducible on exposure to these agents
- VanB - lower level resistance, inducible by vancomycin but strains may remain susceptible to teicoplanin
- VanC - least clinically important, resistance only to vancomycin, constitutive resistance
The development and use of novel antibiotics such as linezolid and daptomycin is expected to delay, but not halt, the emergence of bacteria resistant to all available antibiotics.
References
- ^ a b c d Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006. ISBN 0-9757919-2-3
- ^ Sivagnanam S, Deleu D. Red man syndrome. Crit Care 2003;7(2):119120. PMID 12720556. (full text)
- ^ a b Cantu TG, Yamanaka-Yuen NA, Lietman PS. Serum vancomycin concentrations: reappraisal of their clinical value. Clin Infect Dis 1994;19(6):1180-2. PMID 8038306
- ^ Moellering RC Jr. Monitoring serum vancomycin levels: climbing the mountain because it is there? Clin Infect Dis 1994;18(4):544-6. PMID 8038307
- ^ Karam CM, McKinnon PS, Neuhauser MM, Rybak MJ. Outcome assessment of minimizing vancomycin monitoring and dosing adjustments. Pharmacotherapy 1999;19(3):257-66. PMID 10221365
- ^ Smith TL, Pearson ML, Wilcox KR, Cruz C, Lancaster MV, Robinson-Dunn B, et al. Emergence of vancomycin resistance in Staphylococcus aureus. Glycopeptide-Intermediate Staphylococcus aureus Working Group. N Engl J Med 1999;340(7):493-501. PMID 10021469
- ^ McDonald LC, Killgore GE, Thompson A, et al. Emergence of an epidemic, toxin gene variant strain of Clostridium difficile responsible for outbreaks in the United States between 2000 and 2004. N Engl J Med 2005;353:2433-2441. PMID 16322603
See also
External link
- Vancomycin information created by Princeton University students
- Vancomycin information site and forum




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xxx
216.73.xxx.xxx
 Server Time:
Server Time: