  |
|
| Discovery | |
|---|---|
| Discovered by | Ranajit Ghosh |
| Discovered in | 1952 |
| Chemical characteristics | |
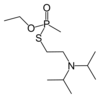
| Chemical name | O-ethyl S-(2-diisopropylaminoethyl)
methylphosphonothioate |
| Chemical family | Organophosphorus compound |
| Chemical formula | C11H26NO2PS |
| NFPA Rating |
|
| Boiling point | 298°C (568.4°F) |
| Freezing/melting point | −50°C (−58°F) |
| Vapor pressure | 0.007 mmHg (? Pa) at 25 °C |
| Vapor Density (Air=1) | 9.2 |
| Liquid density | 1.0083 g cm−3 |
| Specific gravity | 1.0113 |
| Appearance and color | Colorless |
The VX nerve agent is the most well-known of the V-series of nerve agents. Its chemical name is O-ethyl-S-[2(diisopropylamino)ethyl] methylphosphonothioate and its molecular formula is C11H26NO2PS.
The only countries known to possess VX are the United States, United Kingdom, Russia, France and Syria. VX agent is considered an area denial weapon due to its physical properties.
With its high viscosity and low volatility VX has the texture and feel of high-grade motor oil. This makes it especially dangerous, as it has a high persistence in the environment. It is odorless and tasteless, and can be distributed as a liquid or, through evaporation, into small amounts of vapor. It works as a nerve agent by blocking the function of the enzyme acetylcholinesterase. Normally, an electric nerve pulse would cause the release of acetylcholine over a synapse that would stimulate muscle contraction. The acetylcholine is then broken down to non-reactive substances (acetic acid and choline) by the acetylcholinesterase enzyme. If more muscle tension is needed the nerve must release more acetylcholine. VX blocks the action of acetylcholinesterase, thus resulting in sustained contractions of all the muscles in the body. Sustained contraction of the diaphragm muscle causes death by asphyxiation.
Often regarded as the deadliest nerve agent created to date, as little as 200 micrograms is enough to kill an average person, depending on method of absorption. Death can be avoided if the appropriate antidote is injected immediately after exposure. The most commonly used antidote is atropine and pralidoxime which is issued for military personnel in the form of an autoinjector. Standard chemical agent resistance pills are also effective. Atropine works by binding and blocking a subset of acetylcholine receptors (known as muscarinic acetylcholine receptor, mAchR), so that the build up of acetylcholine produced by loss of the acetylcholinesterase function can no longer affect their target. This prevents involuntary muscle actions so that muscles like the diaphragm are not in constant contraction. The injection of pralidoxime regenerates bound acetylcholinesterase.
Contents |
Synthesis
VX is produced via the "Transester Process". This entails a complex chemical transition whereby phosphorus trichloride is methylated to produce methyl phosphonous dichloride. The resulting material is reacted with ethanol to form a diester. This is then transesterified to produce the immediate precursor of VX. Finally, the immediate precursor is reacted with sulfur to form V-agent.
Nerve Agent VX can also be delivered in binary chemical weapons which mix in-flight to form the agent prior to release. Binary VX is referred to as VX2, and is created by mixing O-(2-diisopropylaminoethyl) O'-ethyl methylphosphonite (Agent QL) with elemental sulfur (Agent NE) as is done in the BIGEye aerial chemical bomb. It may also be produced by mixing with sulfur compounds, as with the liquid dimethyl polysulfide mixture (Agent NM) in the cancelled XM-768 8-inch binary projectile program.
History
The chemist Ranajit Ghosh discovered the V-series nerve agents at the Government research establishment at Porton Down, England in 1952; VX was passed over in favour of continuing with sarin as their chemical weapon of choice. The United Kingdom unilaterally renounced chemical and biological weapons in 1956. In 1958 the British government traded their research on VX technology with the United States of America in exchange for information on thermonuclear weapons. The US then went into production of large amounts of VX in 1961.
The US later destroyed all of its stockpiles of the deadly nerve agent (by incineration at Johnston Island in the South Pacific), as mandated by the US accession to the Chemical Weapons Convention. Earlier, pre-treaty disposal included the US Army's CHASE (Cut Holes And Sink 'Em) program, in which old ships were filled with chemical weapons stockpiles and then scuttled. CHASE 8 was conducted on June 15, 1967, in which the S.S. Cpl. Eric G. Gibson was filled with 7,380 VX rockets and scuttled in 7,200 feet of water, off the coast of Atlantic City, New Jersey. The long-term environmental ramifications of exposing large quantities of VX to seawater and marine life could pose a grave danger, but are ultimately unknown.
The US is also destroying chemical weapons stockpiles containing VX in nine other locations, one of which is in Russia. On June 12, 2005, it was reported that more than 250,000 US gallons (950 m³) of the chemical weapon are stored at the Newport Chemical Depot in Newport, Indiana, about 30 miles (50 km) north of Terre Haute, Indiana. The VX is in the process of being hydrolyzed to much less toxic byproducts using concentrated caustic solution. The VX hydrolysate produced will contain mainly a phosphonate ester and a thiolamine, with 20 parts per billion or less of residual VX. (Interestingly, 20 ppb is the level of VX in water that is considered permissible for drinking by US combat troops.) The current plan is to truck the hydrolysate from Indiana to the DuPont Chambers Works Secure Environmental Facility at Deepwater, NJ where it will be further treated to destroy the phosphonate ester and the thiolamine, and dumped into the Delaware River.[1] The US Army is awaiting final approval of the plan from the Centers for Disease Control and the Environmental Protection Agency. The governors of Delaware, New Jersey, Pennsylvania, and New York have opposed this plan and the New Jersey Governor Codey instructed the New Jersey Department of Transportation to deny entry to any trucks carrying the hydrolysate to the Deepwater facility. Prior to the current plan, it had been proposed that the hydrolysate be dumped into the Great Miami River, a tributary of the Ohio River, near Dayton, Ohio but the community there successfully defeated the proposal.
VX hydrolysis began on May 5 2005 and as of June 12 the facility had destroyed 2,894 US gallons (11 m³) of VX. A contained spill of 30 US gallons (100 L) drew attention to the disposal process, but authorities said no agent was released and no one was injured in the spill.
VX was synthesised and used for several murders and attempted murders by the Aum Shinrikyo cult in Japan in 1994 and 1995.[2]
References
- ^ Montgomery, Jeff. "Army backs VX disposal at N.J. facility", The News Journal, 2006-04-26, p. A2.
- ^ Chronology of Aum Shinrikyo's CBW Activities (pdf).




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 131.108.xx.xxx
131.108.xx.xxx
 Server Time:
Server Time: