 |
|
|
Suxamethonium chloride
|
|
| Systematic (IUPAC) name | |
|
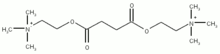
2,2'-[(1,4-dioxobutane-1,4-diyl)bis(oxy)]bis (N,N,N-trimethylethanaminium) |
|
| Identifiers | |
| CAS number | 306-40-1 |
| ATC code | M03AB01 |
| PubChem | 5314 |
| DrugBank | APRD00159 |
| Chemical data | |
| Formula | C14H30N2O4 |
| Mol. weight | 290.399 g/mol |
| Pharmacokinetic data | |
| Bioavailability | NA |
| Metabolism | By pseudocholinesterase, to succinylmonocholine and choline |
| Excretion | Renal (10%) |
| Therapeutic considerations | |
| Pregnancy cat. | A(AU) C(US) |
| Legal status | POM(UK) ℞-only(US) |
| Routes | Intravenous |
Suxamethonium chloride (also known as succinylcholine, or scoline) is a white crystalline substance, it is odourless and highly soluble in water. The compound consists of two acetylcholine molecules that are linked by their acetyl groups. Suxamethonium is sold under several trademark names such as Anectine®, and may be referred to as "sux" for short.
Suxamethonium acts as a depolarizing muscle relaxant. It imitates the action of acetylcholine at the neuromuscular junction, but it is not degraded by acetylcholinesterase but by pseudocholinesterase, a plasma cholinesterase. This hydrolysis by pseudocholinesterase is much slower than that of acetylcholine by acetylcholinesterase.
Contents |
Phase 1 block
There are two phases to the blocking effect of suxamethonium. The first is due to the prolonged stimulation of the acetylcholine receptor results first in disorganized muscle contractions (fasciculations, considered to be a side effect as mentioned below), as the acetylcholine receptors are stimulated. On stimulation, the acetylcholine receptor becomes a general ion channel, so there is a high flux of potassium out of the cell, and of sodium into the cell, resulting in a membrane potential less than the action potential. So, after the initial firing, the cell remains refractory.
Phase 2 block
On continued stimulation, the acetylcholine receptors become desensitised and close. This means that new acetylcholine signals do not cause an action potential; and the continued binding of suxamethonium is ignored. This is the principal anaesthetic effect of suxamethonium, and wears off as the suxamethonium is degraded, and the acetylcholine receptors return to their normal configuration. The side effect of hyperkalaemia is because the acetylcholine receptor is propped open, allowing continued flow of potassium ions into the extracellular fluid. A typical increase of potassium ion serum concentration on administration of suxamethonium is 0.5 mmol per litre, whereas the normal range of potassium is 3.5 to 5 mmol per litre: a significant increase which results in the other side-effects of ventricular fibrilation due to reduced to action potential initiation in the heart.
Medical uses
Its medical uses are limited to short-term muscle relaxation in anesthesia and intensive care, usually for facilitation of endotracheal intubation. Despite its many undesired effects on the circulatory system and skeletal muscles (including malignant hyperthermia, a rare but life-threatening disease), it is perennially popular in emergency medicine because it arguably has the fastest onset and shortest duration of action of all muscle relaxants. Both are major points of consideration in the context of trauma care, where paralysis must be induced very quickly and the use of a longer-acting agent might mask the presence of a neurological deficit.
A single intravenous dose of 1.0 to 1.5 milligrams per kilogram of body weight for adults or 2.0 milligrams per kilogram for pediatrics will cause flaccid paralysis within a minute of injection. For intramuscular injection higher doses are used and the effects last somewhat longer. Suxamethonium is quickly degraded by plasma cholinesterase and the duration of effect is usually in the range of a few minutes. When plasma levels of cholinesterase are greatly diminished or an atypical form of cholinesterase is present (an otherwise harmless inherited disorder), paralysis may last much longer.
Side effects
Side effects include fasciculations, muscle pains, acute rhabdomyolysis with hyperkalemia, transient ocular hypertension, and changes in cardiac rhythm including bradycardia, cardiac arrest, and ventricular dysrhythmias. In children with unrecognized neuromuscular diseases, a single injection of succinylcholine can lead to massive release of potassium from skeletal muscles with cardiac arrest.
Succinylcholine does not produce unconsciousness or anesthesia, and its effects may cause considerable psychological distress while simultaneously making it impossible for a patient to communicate. For these reasons, administration of the drug to a conscious patient is strongly contraindicated, except in necessary emergency situations.
This drug has occasionally been used as a paralyzing agent for executions by lethal injection, although pancuronium bromide is the preferred agent today because of its longer duration of effect and its absence of fasciculations as a side effect. It has also been used for murder.[1]
Succinylcholine is the drug that is suspected to have been used to murder Nevada State Controller Kathy Augustine.




 216.73.216.81
216.73.216.81 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xx
216.73.xxx.xx
 Server Time:
Server Time: