 |
|
|
Raloxifene
|
|
| Systematic (IUPAC) name | |
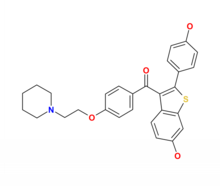
| [6-hydroxy-2-(4-hydroxyphenyl)- benzothiophen-3-yl]- [4-[2-(1-piperidyl)ethoxy]phenyl] -methanone | |
| Identifiers | |
| CAS number | 84449-90-1 |
| ATC code | G03XC01 |
| PubChem | 5035 |
| DrugBank | APRD00400 |
| Chemical data | |
| Formula | C28H27NO4S |
| Mol. weight | 473.584 g/mol |
| Pharmacokinetic data | |
| Bioavailability | 2% |
| Protein binding | 95% |
| Metabolism |
Hepatic glucuronidation CYP system not involved |
| Half life | 27.7 hours |
| Excretion | Fecal |
| Therapeutic considerations | |
| Pregnancy cat. | X(AU) X(US) |
| Legal status | ℞ Prescription only |
| Routes | Oral |
Raloxifene is an oral selective estrogen receptor modulator which is used in the prevention of osteoporosis in postmenopausal women. It was announced on April 17, 2006, that raloxifene is as effective as tamoxifen in reducing the incidence of breast cancer in certain high risk groups of females, [1] [2] though with a reduced risk of thromboembolic events and cataracts in patients taking raloxifene versus those taking tamoxifen.[1] It has not been approved by the FDA for this use, and there has been criticism in the mainstream oncology press of the way that the information was released.[2] There has been some confusion in the lay media about the meaning of the trial results. There is no specific clinical evidence for the use of raloxifene in the adjuvant treatment of breast cancer over established drugs such as tamoxifen or anastrozole.
Raloxifene is produced by Eli Lilly Pharmaceuticals and is sold under the brand name Evista®.
SERMs mimic estrogen in some tissues and have anti-estrogen activity in others. Other SERMs, such as Pfizer's lasofoxifene and Wyeth's bazedoxifene are in the late stages of clinical development.
Contents |
Description
Raloxifene hydrochloride (HCl) has the empirical formula C28H27NO4S•HCl, which corresponds to a molecular weight of 510.05 g/mol. Raloxifene HCl is an off-white to pale-yellow solid that is very slightly soluble in water.
Indication
Raloxifene is indicated for the treatment and prevention of osteoporosis in postmenopausal women.
For either osteoporosis treatment or prevention, supplemental calcium and/or vitamin D should be added to the diet if daily intake is inadequate.
Recently it was shown that raloxifene is a very potent drug for prevention of breast cancer. In a recent clinical trial, the drug was shown to be as effective as tamoxifen for breast cancer prevention with lesser side effects[3].
Contraindications and Precautions
Raloxifene is contraindicated in lactating women or women who are or may become pregnant, in women with active or past history of venous thromboembolic events, including deep vein thrombosis, pulmonary embolism, and retinal vein thrombosis and in women known to be hypersensitive to raloxifene.
Adverse Reactions
Common adverse events considered to be drug-related were hot flashes and leg cramps.
Raloxifene may infrequently cause serious blood clots to form in the legs, lungs, or eyes. Other reactions experienced include leg swelling/pain, trouble breathing, chest pain, vision changes. Eli Lilly, the manufacturer of raloxifene, recently issued a warning that use of raloxifene may be associated with increased risk of death from stroke [4].
References
- ^ Vogel, Victor, Joseph Constantino, Lawrence Wickerman et al.. "Effects of Tamoxifen vs. Raloxifene on the Risk of Developing Invasive Breast Cancer and Other Disease Outcomes". The Journal of the American Medical Association 295 (23): 2727-2741.
- ^ (2006) "A STARring role for raloxifene?". Lancet Oncol 7 (6): 443. PMID 16750489.
- Heringa M (2003). "Review on raloxifene: profile of a selective estrogen receptor modulator.". Int J Clin Pharmacol Ther 41 (8): 331-45. PMID 12940590.
- Barrett-Connor E. "Raloxifene: risks and benefits.". Ann N Y Acad Sci 949: 295-303. PMID 11795366.




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xxx
216.73.xxx.xxx
 Server Time:
Server Time: