| Potassium iodide | |
|---|---|
 |
|
| General | |
| Systematic name | Potassium iodide |
| Other names | Kalium iodide, knollide, potide |
| Molecular formula | KI |
| Molar mass | 166.00 g/mol |
| Appearance | white crystalline solid |
| CAS number | [7681-11-0] |
| Properties | |
| Density and phase | 3.13 g/cm3, solid |
| Solubility in water | 128 g/100 ml (6 °C) |
| Melting point | 681 °C (954 K) |
| Boiling point | 1330 °C (1600 K) |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | Slightly hazardous |
| NFPA 704 |

0
1
0
|
| R/S statement | R: 36, 38, 42-43, 61 S: 26, 36-37, 39, 45 |
| RTECS number | TT2975000 |
| Related compounds | |
| Other anions |
potassium bromide potassium chloride |
| Other cations |
lithium iodide sodium iodide rubidium iodide caesium iodid |
|
Except where noted otherwise, data are given
for materials in their standard state (at 25 °C, 100 kPa) |
|
Potassium iodide is a white crystalline salt with chemical formula KI, used in photography and radiation treatment. It finds widespread application as an iodide source because it is less hygroscopic than sodium iodide, making it easier to work with. KI can turn yellow upon heating in air or upon standing in moist air for long periods, because of oxidation of the iodide to iodine.
Contents |
Chemical properties
Potassium iodide behaves as a simple ionic salt, K+I−. Since the iodide ion is a mild reducing agent, I− is easily oxidised to I2 by powerful oxidising agents such as chlorine:
2 KI(aq) + Cl2(aq) → 2 KCl + I2(aq)
Even air will oxidize iodide as evidenced by the observation of a purple extract when KI is rinsed with dichloromethane. Under acidic conditions, KI is oxidised even more easily, due to the formation of hydroiodic acid (HI), which is a powerful reducing agent.
KI forms I3− when combined with elemental iodine.
-
- KI(aq) + I2(s) → KI3(aq)
Unlike I2, I3− salts can be highly water-soluble. I2 and I3− have virtually identical redox potentials (0.535 and 0.536 V vs NHE, respectively), i.e. they are both mild oxidants relative to H2. Therefore, this reaction allows the iodine to be used in aqueous solutions for redox titrations.
Potassium iodide also serves in some organic reactions as a source of iodide ion (see "uses" below).
Preparation
Potassium iodide may be prepared by the reaction of a potassium base with hydroiodic acid, for example:
HI + KHCO3 → KI + H2O(l) + CO2(g)
Alternatively iron(II) iodide, prepared using scrap iron and iodine (made from iodide rich brines or from Chile saltpeter, can be treated with potassium carbonate:
FeI2 + K2CO3 → 2 KI + FeCO3
Uses
Potassium iodide is used in photography, in the preparation of silver(I) iodide for high speed photographic film:
KI(aq) + AgNO3(aq) → AgI(s) + KNO3(aq)
Potassium iodide is also added to table salt in small quantities to make it "iodized". In a saturated solution, it is also used as an expectorant to treat lung congestion.
KI is often used as a source of iodide ion in organic synthesis. A useful application is in the preparation of aryl iodides from arenediazonium salts[5], for example:
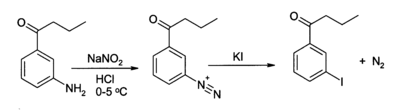
Saturated solution of potassium iodide is also used as treatment for sporotrichosis, a fungal infection.
In medical use, it can also serve as an antiseptic for people suffering from sore throat. The dose is 0.5g-1.0g in 100mL, with the accompany of iodine (0.5g-1.0g in 100mL).
Role of potassium iodide in radiological emergency preparedness

Potassium iodide may also be used to protect the thyroid from radioactive iodine in the event of an accident or attack at a nuclear power plant, or other nuclear attack, especially where a nuclear reactor is breached and the volatile radionuclides, which contain significant amount of 131I, are released into the environment. Radioiodine is a particularly dangerous radionuclide because the body concentrates it in the thyroid gland. Potassium iodide cannot protect against other causes of radiation poisoning, however, nor can it provide any degree of protection against a dirty bomb unless the bomb happens to contain a significant amount of radioactive iodine. In case of a nuclear emergency, iodine used for the cleaning of wounds should not be ingested. It is a poison.
| Age | KI in mg | KIO3 in mg |
|---|---|---|
| Over 12 years old | 130 | 170 |
| 3 - 12 years old | 65 | 85 |
| 1 - 36 months old | 32 | 42 |
| < 1 month old | 16 | 21 |
Precautions
Mild irritant, wear gloves. Chronic overexposure can have adverse effects on the thyroid.
References
- N. N. Greenwood, A. Earnshaw, Chemistry of the Elements, Pergamon Press, Oxford, UK, 1984.
- Handbook of Chemistry and Physics, 71st edition, CRC Press, Ann Arbor, Michigan, 1990.
- The Merck Index, 7th edition, Merck & Co., Rahway, New Jersey, 1960.
- H. Nechamkin, The Chemistry of the Elements, McGraw-Hill, New York, 1968.
- (a) L. G. Wade, Organic Chemistry, 5th ed., pp. 871-2, Prentice Hall, Upper Saddle RIver, New Jersey, 2003. (b) J. March, Advanced Organic Chemistry, 4th ed., pp. 670-1, Wiley, New York, 1992.
- World Health Organization, Guidelines for Iodine Prophylaxis following Nuclear Accidents, Update 1999
External links
- R05CA02
- KI4U Potassium Iodide information relating to a nuclear emergency.
- World Health Organization's guidelines for iodine prophylaxis following a nuclear accident




 216.73.216.81
216.73.216.81 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xx
216.73.xxx.xx
 Server Time:
Server Time: