 |
|
|
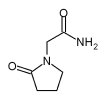
Piracetam
|
|
| Systematic (IUPAC) name | |
| 2-oxo-1-pyrrolidineacetamide | |
| Identifiers | |
| CAS number | 7491-74-9 |
| ATC code | N06BX03 |
| Chemical data | |
| Formula | C6H10N2O2 |
| Mol. weight | 142.156 g/mol |
| Pharmacokinetic data | |
| Bioavailability | ~100 % |
| Half life | 4-5 hours |
| Excretion | Urinary |
| Therapeutic considerations | |
| Legal status |
UK: POM; legal to import Orphan drug |
| Routes | Oral and parenteral |
Piracetam (brand name: Nootropil®, Myocalm®), is a cerebral function regulating drug which is claimed to be able to enhance cognition as well as slow down brain aging. Piracetam's chemical name is 2-oxo-pyrrolidone, or 2-oxo-1-pyrrolidine acetamide. Piracetam is a cyclic derivative of GABA. It is one of the racetams, and is similar to the amino acid pyroglutamate. Though rare in the United States, Piracetam is commonly prescribed in Europe for a variety of conditions.
Contents |
Effects
Several meta-reviews of literature on Piracetam indicate that Piracetam increases performance on a variety of cognitive tasks among dyslexic children, though this may reflect its enhancement of cross-hemispheric communication and of cognitive function in general, rather than a specific improvement in whatever causes dyslexia. Piracetam also seems to inhibit brain damage caused by a variety of factors including hypoxia and excessive alcohol consumption.
Piracetam has been studied in an extensive number of clinical experiments, mostly focusing on dyslexic children, and some believe that understanding the mechanism it works through can teach us about the role of inter-hemispheric communication in the brain.
Mechanisms of action
The mechanisms of action of Piracetam are quite broad. Piracetam is understood to work by stimulating the cerebral cortex as well as by increasing the rate of metabolism and the energy levels of neurons. It possibly facilitates movement of information between the brain's two hemispheres via the corpus callosum, and improves the function of the neurotransmitter acetylcholine via muscarinic cholinergic (ACh) receptors which are implicated in memory processes. Furthermore, Piracetam may have an effect on NMDA glutamate receptors which are involved with learning and memory processes. Finally, Piracetam may exert its global effect on brain neurotransmission via modulation of ion channels (i.e., Ca2+, K+).
History
Piracetam was first synthesized in 1964 by scientists at the Belgian pharmaceutical company UCB led by Dr Corneliu E. Giurgea. The drug was the first of the so-called nootropics ("smart drugs" or "cognitive enhancers"), that is, substances which purportedly enhance mental performance. The term nootropic was coined by Giurgea. Nootropil was launched clinically by UCB in the early 1970s and remains an important product of that company in Europe.
Approval and usage
Piracetam is primarily used in Europe. Piracetam is legal to import into the United Kingdom for personal use with or without prescription as with other prescription-only drugs. As of June of 2006, piracetam is not regulated in the United States (it is neither a controlled substance nor a prescription drug).
Dosage
Piracetam is usually supplied in 800 mg tablets or capsules. The recommended dosage varies based on the indication, usually ranging from 1.6-9.6 grams daily (2-12 pills daily). Some people report faster results when taking 1-2 pills every hour for 4-6 hours or taking 4-8 pills at once for the first few days to notice an effect.
It has been studied up to 45 grams daily without major side effects.
It has no known LD-50 in humans when taken orally.
In the US, and possibly other countries in which Piracetam is unregulated, it is often sold in bulk as a powder. It is up to the user to decide how they want to ingest said powder; orange juice is frequently cited as a good mixer to mask the taste.
Contraindications
Piracetam is contra-indicated in patients with severe renal impairment (renal creatinine clearance of less than 20 ml per minute), hepatic impairment and to those under 16 years of age. It is also contraindicated in patients with cerebral haemorrhage and in those with hypersensitivity to piracetam, other pyrrolidone derivatives or any of the excipients .
Special warnings and precautions for use
Due to the effect of piracetam on platelet aggregation, caution is recommended in patients with underlying disorders of haemostasis, major surgery or severe haemorrhage.
Abrupt discontinuation of treatment should be avoided as this may induce myoclonic or generalised seizures in some myoclonic patients.
As piracetam is almost exclusively excreted by the kidneys caution should be exercised in treating patients with known renal impairment. In renally impaired and elderly patients, an increase in terminal half-life is directly related to renal function as measured by creatinine clearance. Dosage adjustment is therefore required in those with mild to moderate renal impairment and elderly patients with diminished renal function.
Undesirable effects
Following adverse experiences (very rare usually occurring in 1 out of 1,000 people) were reported for piracetam with a statistically significantly higher incidence than placebo. Incidences are given for piracetam versus placebo treated patients.
Central and peripheral nervous system disorders:
hyperkinesia (1.72 versus 0.42 %)
Metabolic and nutritional disorders: weight gain (1.29
versus 0.39 %)
Psychiatric disorders: nervousness (1.13 versus 0.25 %),
somnolence (0.96 versus 0.25 %), depression (0.83 versus
0.21 %)
Body as a whole - general disorders: asthenia (0.23 versus
0.00 %)
Post-marketing experiences have reported the following
undesirable effects:
Ear and labyrinth (inner ear) disorders: vertigo
Gastrointestinal disorders: abdominal pain, upper abdominal
pain, diarrhoea, nausea, vomiting
Immune system disorders: anaphylactoid reaction,
hypersensitivity, multiple chemical sensitivity
Nervous system disorders: ataxia, impaired balance,
aggravated epilepsy, headache, insomnia, somnolence
Psychiatric disorders: agitation, anxiety, confusion,
hallucination
Skin and subcutaneous tissue disorders: angioneurotic oedema,
dermatitis, pruritus, urticaria, rash
References
- SID 7848976 -- PubChem Substance Summary. The PubChem Project. National Center for Biotechnology Information. Retrieved on 7 December 2005.
- UCB Pharma Limited (2005). Nootropil 800mg & 1200mg Tablets and Solution. electronic Medicines Compendium. Datapharm Communications. Retrieved on 8 December 2005.
See also
External links
- Erowid Piracetam Vault
- Erowid Piracetam FAQ
- Collection of Scientific Abstracts on Piracetam
- Piracetam (Nootropyl) by Ward Dean, M.D., and John Morgenthaler
- Nootropics - Reviewing The Smart-Drugs, By James South, MA
- Piracetam - The Original Nootropic, By James South, MA
- The Cognitive Enhancement Research Institute
- Neopharmacology.com - Informational Resource
- Brain Research and Information Network B.R.A.I.N.




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 113.178.xx.xxx
113.178.xx.xxx
 Server Time:
Server Time: