 |
|
 |
|
|
Paroxetine
|
|
| Systematic (IUPAC) name | |
|
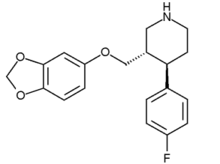
(3S-trans)-3-((1,3-Benzodioxol-5-yloxy)methyl)- 4-(4-fluorophenyl)-piperidine |
|
| Identifiers | |
| CAS number | 61869-08-7 |
| ATC code | N06AB05 |
| PubChem | 43815 |
| DrugBank | APRD00364 |
| Chemical data | |
| Formula | C19H20NFO3 |
| Mol. weight | 374.8 |
| Pharmacokinetic data | |
| Bioavailability | complete absorption from GI, but extensive first-pass-metabolization in the liver; max concentration 4.9 (with meals) to 6.4 hours (fasting) |
| Metabolism | extensive, probable hepatic |
| Half life | 24 hours (range 3-65 hours) |
| Excretion | 66% urine, 37% bile |
| Therapeutic considerations | |
| Licence data | US |
| Pregnancy cat. | D(US) |
| Legal status | ℞ Prescription only |
| Routes | Oral |
Paroxetine or paroxetine hydrochloride is a selective serotonin reuptake inhibitor (SSRI) antidepressant. It was released in 1992 by the pharmaceutical company GlaxoSmithKline and has since become one of the most prescribed antidepressants on the market due to its efficacy in treating depression as well as a spectrum of anxiety disorders ranging from panic attacks to phobias.
Contents |
Trade names
Paroxetine is marketed under several tradenames:
- Aropax or Oxetine in Australia, New Zealand, South Africa, Argentina and Brazil
- Aroxat or Aroxat CR in Chile
- Cebrilin in Latin America
- Deroxat in Switzerland and France
- Optipar in Finland
- Paroxat in Germany and Hungary
- Paxil or Paxil CR in the United States, Canada, Argentina and Brazil
- Pondera in Brazil
- Seroxat in Austria, Belgium, Finland, Greece, Israel, The Netherlands, Poland, Portugal, Singapore, Spain, Turkey, the UK and China.
Indications
Approved
Paroxetine is primarily used to treat the symptoms of depression, obsessive-compulsive disorder (OCD), post-traumatic stress disorder (PTSD), panic disorder, generalized anxiety disorder (GAD), [1] social phobia/social anxiety disorder, [2] and premenstrual dysphoric disorder (PMDD).[3]
It was the first (and as of 2002, the only) antidepressant formally approved in the United States for the treatment of social anxiety disorder, causing it to be sometimes referred to (although inaccurately) as an anti-shyness drug.
Clinical Trials
Trials for Paxil CR have not lasted more than twelve months. The effectiveness of Paxil in major depressive disorders has been proven by two twelve week clinical trials in which the patients either had flexible doses or a placebo. Both of the studies concluded that Paxil is significantly more effective than the placebo control group. For another disorder, three 10-week studies were conducted to prove the effectiveness of Paxil CR on panic disorders. In the first and second studies, Paxil proved consistently better than the placebo. But the third trial Paxil CR failed to have any significant difference to the placebo. For social anxiety disorder, a 12-week trial for adult outpatients was conducted to show Paxil's effectiveness against the disease and prior information of Paxil's nature in the immediate release formulation. The study did not include adolescents with the disorder.
Unapproved/Off-label/Investigational
Paroxetine can also be used in the treatment of premature ejaculation,[4] chronic headache,[5] and bipolar disorder.[6]
Paroxetine has been found to significantly reduce the symptoms of diabetic neuropathy.[7]
There is also evidence that paroxetine may be effective in the treatment of compulsive gambling[8] and hot flashes.[9]
Pharmacology
Paroxetine is the most potent selective serotonin (5-hydroxytryptamine, 5-HT) reuptake inhibitor (SSRI). This activity of the drug on brain neurons is thought to be responsible for its antidepressant effects.
Paroxetine is a phenylpiperidine derivative which is chemically unrelated to the tricyclic or tetracyclic antidepressants. In receptor binding studies, paroxetine did not exhibit significant affinity for the adrenergic (α1, α2, β), dopaminergic, serotonergic (5HT1, 5HT2), or histamine receptors of rat brain membrane. A weak affinity for the muscarinic acetylcholine and noradrenaline receptors was evident. The predominant metabolites of paroxetine are essentially inactive as 5-HT reuptake inhibitors.
Paroxetine controlled release (CR)
Paroxetine controlled release contains a Geomatrix™ tablet that controls the absorption of the drug. Clinical studies show that controlled release tablet provides effective symptom relief with a lower number of patients stopping their treatment due to side effects.[10]
However, the need for an extended release form of paroxetine has not been established, as the FDA indicated that the half-life for the original Paxil was ideal for once-daily dosing, and that a CR version was not needed.
Chemistry
Paroxetine hydrochloride is an odorless, off-white powder, having a melting point range of 120° to 138°C and a solubility of 5.4 mg/mL in water.
Formulations
Paxil / Seroxat (paroxetine) is available in 10, 20, 30, and 40 mg tablets.
Paxil CR (paroxetine extended release) is available in 12.5, 25, and 37.5 mg tablets.
Paxil, Seroxat and Paxil CR are manufactured by GlaxoSmithKline, however a generic is now available under the chemical name paroxetine.
Side effects
General side effects are mostly present during the first 1-4 weeks while the body adapts to the drug. Almost all SSRIs are known to cause either one or more of these symptoms. A person receiving paroxetine treatment may experience a few, all, or none of the following side-effects, and most side-effects will disappear or lessen with continued treatment, though some may last throughout the duration.
- Nausea
Drowsiness
Headache
Changes in weight and appetite
Changes in sexual behaviour
Increased feelings of depression and anxiety (initially)
Dry mouth
Constipation
Diarrhea
Aggressive behavior (esp. in children)
Possible suicidal behavior
Possible congenital malformations
Rash
Restlessness or Akathisia
Itch
Sodium depletion
Changes in urination
Sweating
Muscle weakness
Uncharacteristic levels of aggression
Inability to reach orgasm and other sexual side effects
Individuals experiencing any of the following symptoms should contact their doctor immediately:
- Jaw, neck, and back muscle spasms
Fever, chills, sore throat, or flu-like symptoms
Yellowing of the skin or eyes
Black, tarry stools (this can indicate upper GI bleeding)
Paroxetine and other SSRIs have been shown to cause sexual side effects in some patients, both males and females. Although usually reversible, these sexual side effects can sometimes last for months, years or possibly indefinitely even after the drug has been completely withdrawn. This disorder is known as Post SSRI Sexual Dysfunction.
Discontinuation syndrome
While any psychoactive medication (from caffeine to anti-psychotics) can cause withdrawal symptoms upon discontinuation from acute administration, anecdotal evidence suggests that paroxetine has a higher incidence rate and severity of SSRI discontinuation syndrome than other SSRIs and psychoactive medications. For those experiencing extreme and unusual difficulty discontinuing paroxetine, it is recommended that an SSRI with a longer half-life, such as fluoxetine, be administered for approximately two weeks, then discontinued, to lessen symptoms.[11][12]
Suicidal ideation is a frequently reported experience in those withdrawing from SSRIs.[13] Withdrawal from paroxetine or any other SSRI should be medically supervised.
Footnotes
- ^ Baldwin DS, Anderson IM, Nutt DJ, Bandelow B, Bond A, Davidson JR, den Boer JA, Fineberg NA, Knapp M, Scott J, Wittchen HU (2005). "Evidence-based guidelines for the pharmacological treatment of anxiety disorders: recommendations from the British Association for Psychopharmacology.". Journal of Psychopharmacology 19 (6): 567-596. PMID 16272179.
- ^ D Baldwin, J Bobes, DJ Stein, I Scharwachter and M Faure (1999). "Paroxetine in social phobia/social anxiety disorder. Randomised, double-blind, placebo-controlled study. Paroxetine Study Group". The British Journal of Psychiatry 175: 120-126. PMID 10627793.
- ^ Yonkers KA, Gullion C, Williams A, Novak K, Rush AJ. (1996). "Paroxetine as a treatment for premenstrual dysphoric disorder.". Journal of Clinical Psychopharmacology. 16 (1): 3-8. PMID 8834412.
- ^ Waldinger MD, Hengeveld MW, Zwinderman AH. (1994). "Paroxetine treatment of premature ejaculation: a double-blind, randomized, placebo-controlled study". The American Journal of Psychiatry 151 (9): 1377-1379. PMID 8067497.
- ^ Foster CA, Bafaloukos J. (1994). "Paroxetine in the treatment of chronic daily headache". Headache 34 (10): 587-589. PMID 7843954.
- ^ Sindrup SH, Gram LF, Brosen K, Eshoj O, Mogensen EF (2002). "A randomized trial comparing paroxetine and venlafaxine in the treatment of bipolar depressed patients taking mood stabilizers". Journal of Clinical Psychiatry 63 (6): 508-512. PMID 12088162.
- ^ Vieta E, Martinez-Aran A, Goikolea JM, Torrent C, Colom F, Benabarre A, Reinares M (1999). "The selective serotonin reuptake inhibitor paroxetine is effective in the treatment of diabetic neuropathy symptoms". Pain 42 (2): 135-144. PMID 2147235.
- ^ Kim SW, Grant JE, Adson DE, Shin YC, Zaninelli R (2002). "A double-blind placebo-controlled study of the efficacy and safety of paroxetine in the treatment of pathological gambling". Journal of Clinical Psychiatry 63 (6): 501-507. PMID 12088161.
- ^ Weitzner MA, Moncello J, Jacobsen PB, Minton S. (2002). "A pilot trial of paroxetine for the treatment of hot flashes and associated symptoms in women with breast cancer.". Journal of Pain and Symptom Management 23 (4): 337-345. PMID 11997203.
- ^ Golden RN, Nemeroff CB, McSorley P, Pitts CD, Dube EM. (2002). "Efficacy and tolerability of controlled-release and immediate-release paroxetine in the treatment of depression.". Journal of Clinical Psychiatry 63 (7): 577-584. PMID 12143913.
- ^ Haddad P (2001). "Antidepressant discontinuation syndromes". Drug Saf 24 (3): 183-97. PMID 11347722.
- ^ Quitpaxil.org - Information for persons suffering from Paxil withdrawal syndrome
- ^ Yerevanian B, Koek R, Feusner J, Hwang S, Mintz J (2004). "Antidepressants and suicidal behaviour in unipolar depression". Acta Psychiatr Scand 110 (6): 452-8. PMID 15521830.
External links
- Paroxetine.com - The official paroxetine site
- Paxil Information from Drugs.com
- Paxil Patient Information leaflet, Paxil Patient Information leaflet
- Paxil CR Patient Information leaflet, Paxil CR Patient Information leaflet
- Detailed Paroxetine Consumer Information: Uses, Precautions, Side Effects from medlibrary.org
- The Secrets of Seroxat, BBC Panorama investigation
- Antidepressant Use in Children Soars Despite Efficacy Doubts, Washington Post, April 18, 2004
- Dependence on Antidepressants & Halting SSRIs - protocol for withdrawal
- NIH Expert Panel Report on the reproductive and developmental toxicology of Prozac (Fluoxetine)
- NIH Monograph on the potential human reproductive and developmental effects of Prozac (Fluoxetine)




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xxx
216.73.xxx.xxx
 Server Time:
Server Time: