| Ozone | |
|---|---|
|
|
| General | |
| Systematic name | Trioxygen |
| Molecular formula | O3 |
| Molar mass | 47.998 g/mol |
| Appearance | bluish colored gas |
| CAS number | [10028-15-6] |
| Properties | |
| Density and phase | 2.144 g/l (0 °C), gas |
| Solubility in water | 0.105 g/100 ml (0 °C) |
| Melting point | 80.7 K, −192.5 °C |
| Boiling point | 161.3 K, −111.9 °C |
| Thermodynamic data | |
|
Standard enthalpy of formation ΔfH°solid |
+142.3 kJ/mol |
|
Standard molar entropy S°solid |
237.7 J.K−1.mol−1 |
| Hazards | |
| EU classification | not listed |
| NFPA 704 | |
|
Except where noted otherwise, data are given
for materials in their standard state (at 25 °C, 100 kPa) |
|
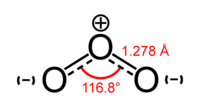
Ozone (O3) is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic species O2. It is present in low concentrations throughout the Earth's atmosphere. It has many industrial and consumer applications as well as being used in ozone therapy.
Ozone, the first allotrope of a chemical element to be described by science, was discovered by Christian Friedrich Schönbein in 1840, who named it after the Greek word for smell (ozein), from the peculiar odor in lightning storms.[1] The odor from a lightning strike is from electrons freed during the rapid chemical changes, not the ozone itself.[2]
Contents |
Physical properties
Undiluted ozone is a pale blue gas at standard temperature and pressure; it forms a dark blue liquid below −112 °C and a violet-black solid below −193 °C [3]. At concentrations found in the atmosphere it is colorless [4]. The concentration above which it can be smelt (odor threshold) is between 0.0076 and 0.036 ppm[5].
Chemistry
Ozone is a powerful oxidizing agent. It is also unstable at high concentrations, decaying to ordinary diatomic oxygen:
- 2 O3 → 3 O2.
This reaction proceeds more rapidly with increasing temperature and decreasing pressure. Ozone will oxidize metals (except gold, platinum, and iridium) to oxides of the metals in their highest oxidation state:
- 2 Cu2+ + 2 H+ + O3 → 2 Cu3+ + H2O + O2
Ozone converts oxides to peroxides:
- SO2 + O3 → SO3 + O2
It also increases the oxidation number of oxides:
- NO + O3 → NO2 + O2
The above reaction is accompanied by chemiluminescence. The NO2 can be further oxidized:
- NO2 + O3 → NO3 + O2
The NO3 formed can react with NO2 to form N2O5:
- NO2 + NO3 → N2O5
Ozone reacts with carbon to form carbon dioxide, even at room temperature:
- C + 2 O3 → CO2 + 2 O2
Ozone does not react with ammonium salts but it reacts with ammonia to form ammonium nitrate:
- 2 NH3 + 4 O3 → NH4NO3 + 4 O2 + H2O
Ozone reacts with sulfides to make sulfates:
- PbS + 4 O3 → PbSO4 + 4 O2
Sulfuric acid can be produced from ozone, either starting from elemental sulfur or from sulfur dioxide:
- S + H2O + O3 → H2SO4
- 3 SO2 + 3 H2O + O3 → 3 H2SO4
All three atoms of ozone may also react, as in the reaction with tin(II) chloride and hydrochloric acid:
- 3 SnCl2 + 6 HCl + O3 → 3 SnCl4 + 3 H2O
In the gas phase, ozone reacts with hydrogen sulfide to form sulfur dioxide:
- H2S + O3 → SO2 + H2O
In an aqueous solution, however, two competing simultaneous reactions occur, one to produce elemental sulfur, and one to produce sulfuric acid:
- H2S + O3 → S + O2 + H2O
- 3 H2S + 4 O3 → 3 H2SO4
Iodine perchlorate can be made by treating iodine dissolved in cold anhydrous perchloric acid with ozone:
- I2 + 6 HClO4 + O3 → 2 I(ClO4)3 + 3 H2O
Solid nitryl perchlorate can be made from NO2, ClO2, and O3 gases:
- 2 NO2 + 2 ClO2 2 O3 → 2 NO2ClO4 + O2
Ozone can be used for combustion reactions and combusting gases in ozone provides higher temperatures than combusting in dioxygen (O2). Following is a reaction for the combustion of carbon subnitride:
- 3 C4N2 + 4 O3 → 12 CO + 3 N2
Ozone can react at cryogenic temperatures. At 77 K (-196 °C), atomic hydrogen reacts with liquid ozone to form a hydrogen superoxide radical, which dimerizes[6]
- H + O3 → HO2 + O
- 2 HO2 → H2O4
Ozonides can be formed, which contain the ozonide anion, O3-. These compounds are explosive and must be stored at cryogenic temperatures. Ozonides for all the alkali metals are known. KO3, RbO3, and CsO3 can be prepared from their respective superoxides:
- KO2 + O3 → KO3 + O2
Although KO3 can be formed as above, it can also be formed from potassium hydroxide and ozone:[7]
- 2 KOH + 5 O3 → 2 KO3 + 5 O2 + H2O
NaO3 and LiO3 must be prepared by action of CsO3 in liquid NH3 on an ion exchange resin containing Na+ or Li+ ions:[8]
- CsO3 + Na+ → Cs+ + NaO3
Treatment with ozone of calcium dissolved in ammonia leads to ammonium ozonide and not calcium ozonide:[9]
- 3 Ca + 10 NH3 + 6 O3 → Ca•6NH3 + Ca(OH)2 + Ca(NO3)2 + 2 NH4O3 + 2 O2 + H2
Ozone can be used to remove manganese from the water, forming a precipitate which can be filtered:
- 2 Mn2+ + 2 O3 + 4 H2O → 2 MnO(OH)2 (s) + 2 O2 + 4 H+
Ozone will also turn cyanides to the one thousand times less toxic cyanates:
- CN- + O3 → CNO- + O2
Finally, ozone will also completely decompose urea:[10]
- (NH2)2CO + O3 → N2 + CO2 + 2 H2O
Ozone in Earth's atmosphere
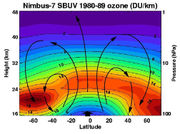
The standard way to express total ozone amounts (the amount of ozone in a vertical column) in the atmosphere is by using Dobson units. Concentrations at a point are measured in parts per billion (ppb) or in μg/m³.
Ozone layer

The highest levels of ozone in the atmosphere are in the stratosphere, in a region also known as the ozone layer between about 10 km and 50 km above the surface. Here it filters out the shorter wavelengths (less than 320 nm) of ultraviolet light (270 to 400 nm) from the Sun that would be harmful to most forms of life in large doses. These same wavelengths are also responsible for the production of vitamin D, which is essential for human health. Ozone in the stratosphere is mostly produced from ultraviolet rays reacting with oxygen:
- O2 + (radiation < 240 nm) → 2 O
- O + O2 → O3
It is destroyed by the reaction with atomic oxygen:
- O3 + O → 2 O2
The latter reaction is catalysed by the presence of certain free radicals, of which the most important are hydroxyl (OH), nitric oxide (NO) and atomic chlorine (Cl) and bromine (Br). In recent decades the amount of ozone in the stratosphere has been declining mostly due to emissions of CFCs and similar chlorinated and brominated organic molecules, which have increased the concentration of ozone-depleting catalysts above the natural background. For more information on stratospheric ozone see Seinfeld and Pandis (1999).
Low level ozone
Low level ozone (or tropospheric ozone) is regarded as a pollutant by the World Health Organisation[11]. It is not emitted directly by car engines or by industrial operations. It is formed by the reaction of sunlight on air containing hydrocarbons and nitrogen oxides that to form ozone directly at the source of the pollution or many kilometers down wind. For more details of the complex chemical reactions that produce low level ozone see tropospheric ozone or Seinfled and Pandis (1998).
Ozone reacts directly with some hydrocarbons such as aldehydes and thus begins their removal from the air, but the products are themselves key components of smog. Ozone photolysis by UV light leads to production of the hydroxyl radical and this plays a part in the removal of hydrocarbons from the air, but is also the first step in the creation of components of smog such as peroxyacyl nitrates which can be powerful eye irritants. The atmospheric lifetime of tropospheric ozone is about 22 days and its main removal mechanisms are being deposited to the ground, the above mentioned reaction giving OH, and by reactions with OH and the peroxy radical HO2· (Stevenson et al, 2006) [12].
As well as having impact on human health (see below) there is also evidence of significant reduction in agricultural yields due to increased ground-level ozone and pollution which interferes with photosynthesis and stunts overall growth of some plant species.[13][14]
Ozone as a greenhouse gas
Although ozone was present at ground level before the industrial revolution, peak concentrations are far higher than the pre-industrial levels and even background concentrations well away from sources of pollution are substantially higher.[15][16] This increase in ozone is of further concern as ozone present in the upper troposphere acts as a greenhouse gas, absorbing some of the infrared energy emitted by the earth. Quantifying the greenhouse gas potency of ozone is difficult as it is not present in uniform concentrations across the globe. However, the most recent scientific review on the climate change (the IPCC Third Assessment Report[17]) suggests that the radiative forcing of tropospheric ozone is about 25% that of carbon dioxide.
Ozone and health
Ozone in air pollution
There is a great deal of evidence to show that high concentrations (ppm) of ozone, created by high concentrations of pollution and daylight UV rays at the earth's surface, can harm lung function and irritate the respiratory system [11][18]. There has also been shown to be a connection between increased ozone caused by thunderstorms and hospital admissions of asthma sufferers [19]. Air quality guidelines such as those from the World Health Organization are based on detailed studies of what levels can cause measurable health effects.
Physiology of ozone
Ozone, along with reactive forms of oxygen such as superoxide, singlet oxygen (see oxygen), hydrogen peroxide, and hypochlorite ions, is naturally produced by white blood cells and other biological systems (such as the roots of marigolds) as a means of destroying foreign bodies. Ozone reacts directly with organic double bonds. Also, when ozone breaks down to dioxygen it gives rise to oxygen free radicals, which are highly reactive and capable of damaging many organic molecules. Ozone has been found to convert cholesterol in the blood stream to plaque (which causes hardening and narrowing of arteries). Moreover, it is believed that the powerful oxidizing properties of ozone may be a contributing factor of inflammation. The cause-and-effect relationship of how the ozone is created in the body and what it does is still under consideration and still subject to various interpretations, since other body chemical processes can trigger some of the same reactions. A team headed by Dr. Paul Wentworth Jr. of the Department of Chemistry at the Scripps Research Institute has shown evidence linking the antibody-catalyzed water-oxidation pathway of the human immune response to the production of ozone. In this system, ozone is produced by antibody-catalyzed production of trioxidane from water and neutrophil-produced singlet oxygen. [20]. See also trioxidane for more on this biological ozone-producing reaction.
Ozone has also been proven to form specific, cholesterol-derived metabolites that are thought to facilitate the build-up and pathogenesis of atherosclerotic plaques (A form of heart disease). These metabolites have been confirmed as naturally occurring in human atherosclerotic arteries and are categorized into a class of secosterols termed “Atheronals”, generated by ozonolysis of cholesterol's double bond to form a 5,6 secosterol as well as a secondary condensation product via aldolization.[21] Volume: Number: Page: 23 DOI:
Safety
Artificial production
Ozone may be formed from O2 by electrical discharges and by action of high energy electromagnetic radiation. Certain electrical equipment generate significant levels of ozone. This is especially true of devices using high voltages, such as laser printers, photocopiers, and arc welders. Electric motors using brushes can generate ozone from repeated sparking inside the unit. Large motors, such as those used by elevators or hydraulic pumps, will generate more ozone than smaller motors.
Industrial production
Ozone used in industry is measured in ppm or mg/L (OSHA exposure limits for example), and percent by mass or weight. Industrially, ozone is produced with short wavelength ultraviolet radiation from a mercury vapor lamp or the application of a high voltage electrical field in a process called cold or corona discharge. The cold discharge apparatus consists of two metal plates separated by an air gap and a high dielectric strength electrical insulator such as borosilicate glass or mica. A high voltage alternating current is applied to the plates and the ozone is formed in the air gap when O2 molecules disassociate and recombine into O3. A faint corona may be present in the air gap, but the voltage is maintained below that which would cause punch-through of the insulator with subsequent arcing and plasma formation.
Laboratory production
In the laboratory ozone can be produced by electrolysis using a 9 volt battery, a pencil graphite rod cathode, a platinum wire anode and a 3M sulfuric acid electrolyte.[22] The half cell reactions taking place are:
- 3 H2O → O3 + 6 H+ + 6 e− ΔEo = − 1.53 V
- 6 H+ + 6 e− → 3 H2 ΔEo = 0 V
- 2 H2O → O2 + 4 H+ + 4 e− ΔEo = −1. 23 V
So that in the net reaction three equivalents of water are converted into one equivalent of ozone and three equivalents of hydrogen. Oxygen formation is a competing reaction.
Applications
Industrial applications
Ozone can be used for bleaching substances and for killing bacteria. Many municipal drinking water systems kill bacteria with ozone instead of the more common chlorine. Ozone does not form organochlorine compounds, but it also does not remain in the water after treatment, so some systems introduce a small amount of chlorine to prevent bacterial growth in the pipes, or may use chlorine intermittently, based on results of periodic testing. Where electrical power is abundant, ozone is a cost-effective method of treating water, as it is produced on demand and does not require transportation and storage of hazardous chemicals. Once it has decayed, it leaves no taste or odor in drinking water. Low level of Ozone is helpful to purify air inside the house. Eliminate mildew and mold build up.
Industrially, ozone or ozonated water is used to
- disinfect water before it is bottled;
- deodorize air and objects, such as after a fire;
- kill bacteria on food or on contact surfaces;
- scrub yeast and mold spores from the air in food processing plants;
- wash fresh fruits and vegetables to kill yeast, mold and bacteria;
- chemically attack contaminants in water (iron, arsenic, hydrogen sulfide, nitrites, and complex organics lumped together as "color");
- provide an aid to flocculation (agglomeration of molecules, which aids in filtration, where the iron and arsenic are removed);
- clean and bleach fabrics (the latter use is patented);
- assist in processing plastics to allow adhesion of inks;
- age rubber samples to determine the useful life of a batch of rubber;
- in surface water treatment plants to eradicate water borne parasites such as Giardia and Cryptosporidium. This process is known as ozonation.
Ozone is a reagent in many organic reactions in the laboratory and in industry. Ozonolysis is the cleavage of an alkene to carbonyl compounds.
Many hospitals in the U.S. and around the world use large ozone generators to decontaminate operating rooms between surgeries. The rooms are cleaned and then sealed airtight before being filled with ozone which effectively kills or neutralizes all remaining bacteria.
Consumer applications
Ozone machines, with or without ionization, are currently used to sanitize (high ozone output) and deodorize non-inhabited rooms, ductwork, vehicles, boats, woodsheds, and buildings.
Some models of air purifiers that also emit low levels of ozone have been sold in the US. These type of air purifiers claim to imitate nature's "filterless" air purifying mechanisms [23] and claim to "sanitize" the air and/or household surfaces. The government successfully sued one company in 1995, ordering them to stop repeating health claims without supporting scientific studies.
Ozonated water is used to launder clothes, sanitize food, drinking water, and surfaces in the home. According to the FDA, it is "amending the food additive regulations to provide for the safe use of ozone in gaseous and aqueous phases as an antimicrobial agent on food, including meat and poultry." Ironically, while ozone is considered an atmospheric pollutant, pollution and smog by the US government, it can actually reduce pollutants like pesticides in fruits and vegetables.[24]
Ozone is used in spas or hot tubs with reduced levels of Chlorine or Bromine for keeping the water free of bacteria. As it does not remain in the water after treatment, it is ineffective at preventing bather cross-contamination, and must be used in conjuction with another sanitizer. Ozone gas is created by an ultraviolet light bulb or corona discharge chip and injected into the plumbing system.
Ozone is also widely used in treatment of water in aquaria and fish ponds. Its use can minimise bacterial growth control parasites and removes or reduce "yellowing" of the water. As the Ozone rapidly decomposes, at correctly controlled levels the application has no effect on the fish.
Pharmaceutical applications
Ozone has a number of medical uses. It can be used to affect the body's antioxidant-prooxidant balance, since the body usually reacts to its presence by producing antioxidant enzymes.[citation needed] Ozone therapy has blossomed into a thriving field of alternative medicine, with a host of claimed applications far above and beyond what has actually been verified by studies .
References
- ^ Today in Science History. Retrieved on 2006-05-10.
- ^ Ozone FAQ. Global Change Master Directory. Retrieved on 2006-05-10.
- ^ Oxygen. WebElements. Retrieved on 2006-09-23.
- ^ Ozone: Helpful or Harmful?. Aerias AQS IAQ Resource Center. Retrieved on 2006-09-23.
- ^ Ozone. Haz-MAP (occupational health database}. Retrieved on 2006-09-23.
- ^ Horvath M., Bilitzky L., & Huttner J., 1985. "Ozone." pg 44-49
- ^ Housecroft & Sharpe, 2005. "Inorganic Chemistry." pg 439
- ^ Housecroft & Sharpe, 2005. "Inorganic Chemistry." pg 265
- ^ Horvath M., Bilitzky L., & Huttner J., 1985. "Ozone." pg 44-49
- ^ Horvath M., Bilitzky L., & Huttner J., 1985. "Ozone." pg 259, 269-270
- ^ a b WHO-Europe reports: Health Aspects of Air Pollution (2003) (PDF)
- ^ Stevenson et al (2006). Multimodel ensemble simulations of present-day and near-future tropospheric ozone. American Geophysical Union. Retrieved on 2006-09-16.
- ^ Rising Ozone Levels Pose Challenge to U.S. Soybean Production, Scientists Say. NASA Earth Observatory (2003-07-31). Retrieved on 2006-05-10.
- ^ Mutters, Randall (March 1999). Statewide Potential Crop Yield Losses From Ozone Exposure. California Air Resources Board. Retrieved on 2006-05-10.
- ^ Tropospheric Ozone in EU - The consolidated report. European Environmental Agency (1998). Retrieved on 2006-05-10.
- ^ Atmospheric Chemistry and Greenhouse Gases. Intergovernmental Panel on Climate Change. Retrieved on 2006-05-10.
- ^ Climate Change 2001. Intergovernmental Panel on Climate Change (2001). Retrieved on 2006-09-12.
- ^ Answer to follow-up questions from CAFE (2004) (PDF)
- ^ Anderson, W., G.J. Prescott, S. Packham, J. Mullins, M. Brookes, and A. Seaton (August 2001). "Asthma admissions and thunderstorms: a study of pollen, fungal spores, rainfall, and ozone". QJM: An International Journal of Medicine 94 (8): 429-433. Retrieved on 2006-09-23.
- ^ Hoffmann, Roald (January 2004). "The Story of O". American Scientist 92 (1): 23. DOI:10.1511/2004.1.23. Retrieved on 2006-10-11.
- ^ Paul Wentworth (November 2003). Evidence for Ozone Formation in Human Atherosclerotic Arteries. Retrieved on 2006-08-03.
- ^ Ibanez, Jorge G., Rodrigo Mayen-Mondragon and M. T. Moran-Moran (October 2005). "Laboratory Experiments on the Electrochemical Remediation of the Environment. Part 7: Microscale Production of Ozone". Journal of Chemical Education 82: 1546. Retrieved on 2006-05-10.
- ^ The Unknown Truth Regarding Ozone!. Retrieved on 16-09-2006.
- ^ lotus Sanitizes Food without Chemicals (2000). Retrieved July 24, 2006.
- Seinfeld, John H.; Pandis, Spyros N (1998). Atmospheric Chemistry and Physics - From Air Pollution to Climate Change. John Wiley and Sons, Inc. ISBN 0-471-17816-0
- Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.). Oxford:Butterworth-Heinemann. ISBN 0-7506-3365-4.
External links
- NASA's Ozone Resource Page
- Paul Crutzen Interview Freeview video of Paul Crutzen Nobel Laureate for his work on decomposition of ozone talking to Harry Kroto Nobel Laureate by the Vega Science Trust.
- NASA's Earth Observatory article on Ozone
- International Day for the Preservation of the Ozone Layer
- International Chemical Safety Card 0068
- NIOSH Pocket Guide to Chemical Hazards
- National Institute of Environmental Health Sciences Ozone Alerts
- Ground-level Ozone Air Pollution — A summary for non specialists by GreenFacts of the above WHO reports.
- NASA Study Links "Smog" to Arctic Warming— NASA Goddard Institute for Space Studies (GISS) study shows the warming effect of ozone in the Arctic during winter and spring.
- EPA Assessment of Effectiveness and Health Consequences of Ozone Generators that are Sold as Air Cleaners




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xxx
216.73.xxx.xxx
 Server Time:
Server Time: