| Muscarine | |
|---|---|
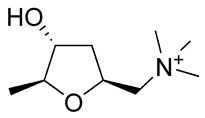
| Chemical name | (2S,4R,5S)-(4-hydroxy-5-methyl-tetrahydrofuran- 2-ylmethyl)-trimethyl-ammonium |
| Chemical formula | C9H20NO2+ |
| Molecular mass | 174.26 g/mol |
| CAS number | 300-54-9 |
| SMILES | O[C@@H]1C[C@@H](C[N+](C)(C)C)O[C@H]1C |
 |
|
Muscarine, L-(+)-muscarine, or muscarin is a natural product found in certain mushrooms, particularly in Inocybe and Clitocybe species. It was first isolated from Amanita muscaria in 1869. It was the first parasympathomimetic substance ever studied and causes profound activation of the peripheral parasympathetic nervous system that may end in convulsions and death. Muscarine has no effects on the central nervous system because it does not cross the blood-brain barrier due to its positively charged nitrogen atom.
Muscarine mimics the action of the neurotransmitter acetylcholine at metabotropic receptors that are also known under the name muscarinic acetylcholine receptors.
Muscarine poisoning is characterized by increased salivation, sweating (perspiration), and tearflow (lacrimation) within 15 to 30 minutes after ingestion of the mushroom. With large doses, these symptoms may be followed by abdominal pain, severe nausea, diarrhea, blurred vision, and labored breathing. Intoxication generally subsides within 2 hours. Deaths are rare, but may result from cardiac or respiratory failure in severe cases. The specific antidote is atropine.
Muscarine is only a trace compound in the fly agaric Amanita muscaria, the pharmacologically more relevant compound from this mushroom is muscimol.
External links
References
- Katzung, Bertam G. Basic and Clinical Pharmacology, 9th ed. (2004). ISBN 0-07-141092-9




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 125.162.xxx.xxx
125.162.xxx.xxx
 Server Time:
Server Time: