 |
|
|
Midazolam
|
|
| Systematic (IUPAC) name | |
|
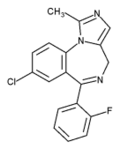
8-chloro-6-(2-fluorophenyl)-1-methyl- 4H-Imidazo(1,5-a)(1,4)benzodiazepine |
|
| Identifiers | |
| CAS number | 59467-70-8 |
| ATC code | N05CD08 |
| PubChem | 4192 |
| DrugBank | APRD00680 |
| Chemical data | |
| Formula | C18H13N3ClF |
| Mol. weight | 325.78 |
| Pharmacokinetic data | |
| Bioavailability | Oral ~36% I.M. 90%+ |
| Metabolism | Hepatic |
| Half life | 1.8-6.4 hours |
| Excretion | Renal |
| Therapeutic considerations | |
| Pregnancy cat. | C (USA) C (Aus) |
| Legal status | Schedule IV(US) |
| Routes | Oral, I.M., I.V., parenteral |
Midazolam, (marketed under brand names Versed®, Hypnovel® and Dormicum®, pronounced mɪˈdæzəlæm) is a drug which is a benzodiazepine derivative. It has powerful anxiolytic, amnestic, hypnotic, anticonvulsant, skeletal muscle relaxant and sedative properties. It is considered a fast-acting benzodiazepine, with a short elimination half-life.
Midazolam was first synthesized in 1976 by Fryer and Walser.
Contents |
Pharmacology
Unlike other benzodiazepines such as diazepam and lorazepam, midazolam is water soluble because the imidazoline ring is open at pH under 4. However, when it is injected, the slightly alkaline (pH about 7.4) environment of the bloodstream causes the imidazoline ring to close, and it becomes much more lipid soluble, facilitating its rapid uptake into nerve tissue. This, and the fact that it has a pKa of 6.15 and is therefore predominantly un-ionised (>90%) at physiological pH, accounts for its rapid onset of action.
Metabolism is by the hepatic Cytochrome p450 enzyme 3A3/3A4. It is hydroxylated to the active metabolite 1-hydroxy-midazolam and then glucuronidated before being renally excreted.
Indications
Midazolam is frequently used (usually in combination with other agents, such as morphine) by anesthesia providers (nurse anesthetists and anesthesiologists) for sedating patients prior to surgery or other invasive medical procedures, such as endoscopy. The patient typically does not actually lose consciousness, but may lose the ability to form memories (anterograde amnesia).
Due to its high potency and fast onset of effects, it is rarely prescribed outside of hospitals. An exception is buccal midazolam, used for the rapid treatment of prolonged seizures. This is an off-label use usage of midazolam, although it has become increasingly common. When using it for this purpose, the drug is squirted slowly between the gums and the inside of the cheek, where it is absorbed directly into the blood stream.
- Intramuscular or intravenous:
- Preoperative sedation, anxiolysis, amnesia,
- Treatment of epileptic seizures.
- Intravenous:
- For sedation, anxiolysis, and amnesia prior to endoscopic procedures. Often used in combination with other CNS depressants.
- For general anesthesia, often in combination with other anesthetic agents.
- Continuous I.V. infusion:
- For sedation of intubated patients in an intensive care setting.
Oral: Syrup formulation is often used for dental preoperative sedation for children.
Dosage
The dosing of midazolam is highly variable depending on other patient factors and other concurrently administered drugs.
- Preoperative anxiolysis: 0.5-2.5 mg.
- Endoscopy: 3-5 mg (rarely 10mg) IV, often paired with short acting opiods in moderate dosage. For example, 50mg pethidine or equivalent dosage of fentanyl (2.5-5 micrograms).
- Status epilepticus: 10mg intranasally or as buccal.
Side effects and abuse potential
Midazolam is categorized as a Schedule IV controlled substance, meaning it has low abuse potential compared to substances such as hydrocodone or oxycodone. As a benzodiazepine it shares similar side effects to other members of this drug family, however it is far less often abused given the ready availability of alternatives, and its primary use in hospital settings only. The mild amnesia caused by this drug is a side effect commonly used for its effect as a 'pre-med' before surgery. Rapid infusion of midazolam may cause transient apnea, and occasionally, respiratory arrest.
Interactions
Midazolam is metabolized almost completely by cytochrome P450-3A4. Vmax in microsomes is reported as 850 pmol/min/mg microsomal protein. Km is reported as 3.7 uMol. Metabolism in the gut wall is reported as nearly equal to metabolism in liver by CYP3A4. Therefore, midazolam will interact with other 3A4 substrates and inhibitors. Grapefruit juice reduces intestinal 3A4 and results in less gut wall metabolism and higher plasma concentrations, which could result in overdose.
Contraindications
Hypersensitivity, acute narrow angle glaucoma, shock, hypotension, head injury, and drug or alcohol use.
Overdose
Symptoms of midazolam overdose include:
- Somnolence (difficulty staying awake)
- Mental confusion
- Hypotension
- Impaired motor functions
- Impaired reflexes
- Impaired coordination
- Impaired balance
- Dizziness
- Coma
In animal models, the oral LD50 of midazolam is 825 mg/kg.
Midazolam overdose is considered a medical emergency and generally requires the immediate attention of medical personnel. The antidote for an overdose of midazolam (or any other benzodiazepine) is flumazenil (Anexate®).
Legal status
Midazolam is a Schedule IV drug under the Convention on Psychotropic Substances.[1]
External links
Notes
References
- EMEA Summary of Product Characteristics: Hypnovel and associated names.
- Clinical Use of Midazolam by John Shou.
- Brevoord J, Joosten K, Arts W, van Rooij R, de Hoog M (2005). "Status epilepticus: clinical analysis of a treatment protocol based on midazolam and phenytoin.". J Child Neurol 20 (6): 476-81. PMID 15996395.
- Wolfe T, Macfarlane T (2006). "Intranasal midazolam therapy for pediatric status epilepticus.". Am J Emerg Med 24 (3): 343-6. PMID 16635708.
- Johnson T, Rostami-Hodjegan A, Goddard J, Tanner M, Tucker G (2002). "Contribution of midazolam and its 1-hydroxy metabolite to preoperative sedation in children: a pharmacokinetic-pharmacodynamic analysis.". Br J Anaesth 89 (3): 428-37. PMID 12402721.
- Prediction of the disposition of midazolam in surgical patients by a physiologically based pharmacokinetic model, Bjorkman, S et al, J Pharm Sci 2001:90(9)1226-1241.
- Merritt P, Hirshman E, Hsu J, Berrigan M (2005). "Metamemory without the memory: are people aware of midazolam-induced amnesia?". Psychopharmacology (Berl) 177 (3): 336-43. PMID 15290003.




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xxx
216.73.xxx.xxx
 Server Time:
Server Time: