It is widely used around the world; since introduction of its earliest versions in the 1970s, over 500 million doses have been used in over 60 countries. As with all vaccinations, long-term effects and efficacy are subject to continuing study. The vaccine is sold by e.g. Merck as M-M-R II[1] and GlaxoSmithKline as Priorix.
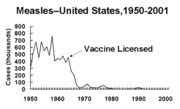
Before the widespread use of a vaccine against measles, its incidence was so high that patients born before 1949 are assumed to have had measles. Today the incidence of measles has fallen to less than one percent of people under the age of 30 in countries with routine childhood vaccination. Measles has a significant complication rate, which includes pneumonitis and encephalitis.
Studies, such as a Centers for Disease Control (CDC) report on the effect of vaccination against measles in Africa between 1996-2002, have shown that vaccination markedly reduces the mortality rate due to measles.[1]
Mumps is another viral disease of childhood that was once very common. A known but relatively rare complication of mumps is sterility in males.
Rubella, otherwise known as German measles, was also very common before the advent of widespread vaccination. The major risk of rubella is if a pregnant woman is infected, her baby may contract congenital rubella from her, which can cause significant congenital defects.

All three diseases are highly contagious.
The MMR vaccine was introduced to induce immunity less painfully than three separate injections at the same time, sooner than at three separate encounters, and more efficiently than either. The incidence and therefore the complications of the three diseases above have declined significantly and this is generally attributed to widespread population vaccination.
Contents |
Adverse Effects
There are a number of adverse effects listed in the product documentation for the MMR vaccine.[1] Additional side effects and variants are reported including: a rash or slight fever for a few days, one to two weeks after receiving the vaccine, occasionally accompanied by a mild swelling of the salivary glands and some aching or swelling of the joints, respectively from the measles, mumps and rubella components, which have differing incubation periods. They are usually mild and temporary, vanishing within a few days. There are rare reports of more serious adverse effects — only about one in every 100,000 vaccinations is reported to have resulted in a severe adverse effect such as encephalitis or meningitis. Acute disseminated encephalomyelitis is a rare severe adverse effect of the vaccine.[2]
In the UK, the vaccine was the subject of controversy after a 1998 paper by Dr. Andrew Wakefield, which claimed to have found a possible link between MMR and the onset of behavioral abnormalities in children. Numerous peer-reviewed studies have since failed to show any correlation. Since its publication, this conclusion of the study has been retracted by ten of Wakefield's co-authors, and his call for parents to boycott the vaccine in favor of single injections one year apart, has been heavily criticized, both on scientific grounds and for triggering a decline in vaccination rates.[2]
Parents may choose to have each of the three components given separately, but the overwhelming medical opinion is that such spacing of injections does not reduce the chance of adverse effects, but increases the opportunity for infection by the two diseases not immunized against first.
Weighing adverse effects and benefits
While there are recognized effects, rarely serious, from each component of the MMR vaccine, from a public health perspective, the benefit to the population outweighs these concerns. The consensus of medical opinion is that the vaccine is very safe.
Development, Formulation and Administration
The component viral strains of MMR vaccine were developed by propagation in animal cells. The live viruses require animal cells as a host for production of more virus.
For example, in the case of mumps and measles viruses, the virus strains were grown in embryonated hens' eggs and chick embryo cell cultures. This produced strains of virus which were adapted for the hens egg and less well-suited for human cells. These strains are therefore called attenuated strains. They are sometimes referred to as neuroattenuated because these strains are less virulent to human neurons than the wild strains.[3] [4]
| Disease Immunized | Component Vaccine | Virus Strain | Propagation Medium | Growth Medium |
|---|---|---|---|---|
| Measles | Attenuvax | Enders' attenuated Edmonston strain [5] | chick embryo cell culture | Medium 199 |
| Mumps | Mumpsvax[6] | Jeryl Lynn (B level) strain[7] | ||
| Rubella | Meruvax II | Wistar RA 27/3 strain of live attenuated rubella virus | WI-38 human diploid lung fibroblasts | MEM (solution containing buffered salts, fetal bovine serum, human serum albumin and neomycin, etc.) |
The virus is extracted from the human albumin growth medium via the Cohn cold ethanol fractionation method. [1]
MMR II is supplied freeze-dried (lyophilized) and contains live viruses. Before injection it is reconstituted with the solvent provided. It is administered by a subcutaneous injection.
Footnotes
- ^ a b c Merck Co. (1990, 1999). "M-M-R II (Measles, Mumps, and Rubella Virus Vaccine Live)". Merck Co..
- ^ http://news.bbc.co.uk/2/hi/health/5118166.stm BBC News, Doctors issue plea over MMR jab, 26 June 2006
- ^ Immunization, Vaccines and Biologicals. World Health Organization (2003).
- ^ "Changes in Mumps Virus Gene Sequence Associated with Variability in Neurovirulent Phenotype".
- ^ Attenuvax Product Sheet (PDF) pp. 1. Merck & Co (September 2002). Retrieved on July 7, 2006.
- ^ Merck Co. (1990, 1999). MUMPSVAX (Mumps Virus Vaccine Live) Jeryl Lynn™ Strain. Merck Co..
- ^ Young ML, Dickstein B, Weibel RE, Stokes J Jr, Buynak EB, Hilleman MR. (1967). "Experiences with Jeryl Lynn strain live attenuated mumps virus vaccine in a pediatric outpatient clinic". Pediatrics. PubMed.
See also
- ProQuad vaccine




 216.73.216.81
216.73.216.81 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xx
216.73.xxx.xx
 Server Time:
Server Time: