|
Interferon alpha 1
|
|
| Identifiers | |
| Symbol(s) | IFNA1 |
| Entrez | 3439 |
| OMIM | 147660 |
| RefSeq | NM_024013 |
| UniProt | P01562 |
| Other data | |
| Locus | Chr. 9 p22 |
|
Interferon alpha 2
|
|
| Identifiers | |
| Symbol(s) | IFNA2 |
| Entrez | 3440 |
| OMIM | 147562 |
| RefSeq | NM_000605 |
| UniProt | P01563 |
| Other data | |
| Locus | Chr. 9 p22 |
|
Interferon alpha 4
|
|
| Identifiers | |
| Symbol(s) | IFNA4 |
| Entrez | 3441 |
| OMIM | 147564 |
| RefSeq | NM_021068 |
| UniProt | P05014 |
| Other data | |
| Locus | Chr. 9 p22 |
|
Interferon alpha 5
|
|
| Identifiers | |
| Symbol(s) | IFNA5 |
| Entrez | 3442 |
| OMIM | 147565 |
| RefSeq | NM_002169 |
| UniProt | P01569 |
| Other data | |
| Locus | Chr. 9 p22 |
|
Interferon alpha 6
|
|
| Identifiers | |
| Symbol(s) | IFNA6 |
| Entrez | 3443 |
| OMIM | 147566 |
| RefSeq | NM_021002 |
| UniProt | P05013 |
| Other data | |
| Locus | Chr. 9 p22 |
|
Interferon alpha 7
|
|
| Identifiers | |
| Symbol(s) | IFNA7 IFNA-J |
| Entrez | 3444 |
| OMIM | 147567 |
| RefSeq | NM_021057 |
| UniProt | P01567 |
| Other data | |
| Locus | Chr. 9 p22 |
|
Interferon alpha 8
|
|
| Identifiers | |
| Symbol(s) | IFNA8 |
| Entrez | 3445 |
| OMIM | 147568 |
| RefSeq | NM_002170 |
| UniProt | P32881 |
| Other data | |
| Locus | Chr. 9 p22 |
|
Interferon alpha 10
|
|
| Identifiers | |
| Symbol(s) | IFNA10 |
| Entrez | 3446 |
| OMIM | 147577 |
| RefSeq | NM_002171 |
| UniProt | P01566 |
| Other data | |
| Locus | Chr. 9 p22 |
|
Interferon alpha 13
|
|
| Identifiers | |
| Symbol(s) | IFNA13 |
| Entrez | 3447 |
| OMIM | 147578 |
| RefSeq | NM_006900 |
| UniProt | P01562 |
| Other data | |
| Locus | Chr. 9 p22 |
|
Interferon alpha 14
|
|
| Identifiers | |
| Symbol(s) | IFNA14 |
| Entrez | 3448 |
| OMIM | 147579 |
| RefSeq | NM_002172 |
| UniProt | P01570 |
| Other data | |
| Locus | Chr. 9 p22 |
|
Interferon alpha 16
|
|
| Identifiers | |
| Symbol(s) | IFNA16 |
| Entrez | 3449 |
| OMIM | 147580 |
| RefSeq | NM_002173 |
| UniProt | P05015 |
| Other data | |
| Locus | Chr. 9 p22 |
|
Interferon alpha 17
|
|
| Identifiers | |
| Symbol(s) | IFNA17 |
| Entrez | 3451 |
| OMIM | 147583 |
| RefSeq | NM_021268 |
| UniProt | P01571 |
| Other data | |
| Locus | Chr. 9 p22 |
|
Interferon alpha 21
|
|
| Identifiers | |
| Symbol(s) | IFNA21 |
| Entrez | 3452 |
| OMIM | 147584 |
| RefSeq | NM_002175 |
| UniProt | P01568 |
| Other data | |
| Locus | Chr. 9 p22 |
|
Interferon beta 1
|
|
| Identifiers | |
| Symbol(s) | IFNB1 IFNB |
| Entrez | 3456 |
| OMIM | 147640 |
| RefSeq | NM_002176 |
| UniProt | P01574 |
| Other data | |
| Locus | Chr. 9 p22 |
|
Interferon beta 3
|
|
| Identifiers | |
| Symbol(s) | IFNB3 |
| Entrez | 3457 |
| OMIM | 147860 |
| RefSeq | [1] |
| UniProt | [2] |
| Other data | |
| Locus | Chr. 8 [3] |
|
Interferon delta
|
|
| Identifiers | |
| Symbol(s) | IFND1 |
| Entrez | [4] |
| RefSeq | [5] |
| UniProt | P37290 |
| Other data | |
| Locus | Chr. [6] |
|
Interferon kappa
|
|
| Identifiers | |
| Symbol(s) | IFNK |
| Entrez | 56832 |
| RefSeq | NM_020124 |
| UniProt | Q9P0W0 |
| Other data | |
| Locus | Chr. 9 p21.2 |
|
Interferon omega 1
|
|
| Identifiers | |
| Symbol(s) | IFNW1 |
| Entrez | 3467 |
| OMIM | 147553 |
| RefSeq | NM_002177 |
| UniProt | P05000 |
| Other data | |
| Locus | Chr. 9 p22 |
Interferons (IFNs) are natural proteins produced by the cells of the immune system of most vertebrates in response to challenges by foreign agents such as viruses, bacteria, parasites and tumor cells. Interferons belong to the large class of glycoproteins known as cytokines.
Contents |
The discovery of interferon
While aiming to develop an improved vaccine for smallpox, two Japanese virologists, Yasu-ichi Nagano and Yasuhiko Kojima working at the then Institute for Infection Disease at the University of Tokyo, noticed that rabbit-skin or testis previously inoculated with UV-inactivated virus exhibited inhibited viral growth when re-infected at the same site with live virus. They hypothesised that this was due to some “facteur inhibiteur” (inhibitory factor), and began to characterise it by fractionation of the UV-irradiated viral homogenates using an ultracentrifuge. They published these findings in 1954 in the French journal now known as “Journal de la Société de Biologie”.[1] While this paper demonstrated that the activity could be separated from the virus particles, it could not reconcile the antiviral activity demonstrated in the rabbit skin experiments, with the observation that the same supernatant led to the production of antiviral antibodies in mice. A further paper in 1958, involving triple-ultracentrifugation of the homogenate demonstrated that the inhibitory factor was distinct from the virus particles, leading to trace contamination being ascribed to the 1954 observations.[2][3]
Meanwhile, the Scottish virologist Alick Isaacs and the Swiss researcher Jean Lindenmann, at the National Institute for Medical Research in London, noticed an interference effect caused by heat-inactivated influenza virus on the growth of live influenza virus in fragments of chick chorioallantoic membrane. They published their results in 1957;[4] in this paper they coined the term ‘interferon’, and today that specific interfering agent is known as a ‘Type I interferon’.
Nagano’s work was never fully appreciated in the scientific community; possibly because it was printed in French, but also because his in vivo system was perhaps too complex to provide clear results in the characterisation and purification of interferon. As time passed, Nagano became aware that his work had not been widely recognised, yet did not actively seek reevaluation of his status in field of interferon research. As such, the majority of the credit for discovery of the interferon goes to Isaacs and Lindenmann, with whom there is no record of Nagano ever having made personal contact.[5]
Types of interferon
Three major classes of interferons have been described for humans, Type I, type II and type III, classified based on the type of receptor through which they signal.
Type I IFN
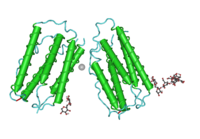
Human type I IFNs comprise a vast and growing group of IFN proteins, designated IFN-α (alpha), IFN-β (beta), IFN-κ (kappa), IFN-δ (delta), IFN-ε (epsilon), IFN-τ (tau), IFN-ω (omega) and IFN-ζ (zeta, also known as limitin)[6].[7] The IFN-α proteins come in 13 subtypes that are called IFNA1, IFNA2, IFNA4, IFNA5, IFNA6, IFNA7, IFNA8, IFNA10, IFNA13, IFNA14, IFNA16, IFNA17, IFNA21. These genes for these IFN-α molecules are found together in a cluster on chromosome 9. Two types of IFN-β have been described, IFN-β1 and IFN-β3[8] (a gene designated IFN-β2 is actually IL-6). IFN-ε, –κ, -τ, and –ζ appear, at this time, to come in a single isoform in humans. IFN-ω, although having only one functional form described to date, has several pseudogenes.[9][10][11][12][13][14][15] Homologous molecules to type I IFNs are found in many species, including most mammals, and some have been identified in birds, reptiles, amphibians and fish species.[16] All type I IFNs bind to a specific cell surface receptor complex known as the IFN-α receptor (IFNAR) that consists of IFNAR1 and IFNAR2 chains.
Type II IFN
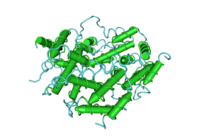
A sole member makes up the type II IFNs that is called IFN-γ (gamma). Mature IFN-γ is an anti-parallel homodimer, which binds to the IFN-γ receptor (IFNGR) complex to elicit a signal within its target cell. IFNGR is made up of two subunits each of molecules designated IFNGR1 and IFNGR2.
Type III IFN
The recently classified type III IFN group consists of three IFN-λ (lamda) molecules called IFN-λ1, IFN-λ2 and IFN-λ3 (also called IL29, IL28A and IL28B respectively).[17] These IFNs signal through a receptor complex consisting of IL10R2 (also called CRF2-4) and IFNLR1 (also called CRF2-12). [18]
The signaling pathway of interferons
While there is evidence to suggest other signaling mechanisms exist, the JAK-STAT signaling pathway is the best-characterised and commonly accepted IFN signaling pathway.
Sources and functions of inteferons
Interferons in general have several effects in common. They are antiviral and possess antioncogenic properties, macrophage and natural killer lymphocyte activation, and enhancement of major histocompatibility complex glycoprotein classes I and II, and thus presentation of foreign (microbial) peptides to T cells. In a majority of cases, the production of interferons is induced in response to microbes such as viruses and bacteria and their products (viral glycoproteins, viral RNA, bacterial endotoxin, bacterial flagella, CpG DNA), as well as mitogens and other cytokines, for example interleukin 1, interleukin 2, interleukin-12, tumor necrosis factor and colony-stimulating factor, that are synthesised in the response to the appearance of various antigens in the body. Their metabolism and excretion take place mainly in the liver and kidneys. They rarely pass the placenta and the blood-brain barrier.
Type I interferons
IFN-α and IFN-β are secreted by many cell types including lymphocytes (NK cells, B-cells and T-cells), macrophages, fibroblasts, endothelial cells, osteoblasts and others. They stimulate both macrophages and NK cells to elicit and anti-viral response, and are also active against tumors. IFN-ω is released by leukocytes at the site of viral infection or tumors.
Type II interferons
IFN-γ is involved in the regulation of the immune and inflammatory responses; in humans, there is only one type of interferon-gamma. It is produced in activated T-cells and natural killer cells. IFN-γ has some anti-viral and anti-tumor effects, but these are generally weak. However, this cytokine potentiates the effects of the type I IFNs. IFN-γ released by Th1 cells recruits leukocytes to a site of infection, resulting in increased inflammation. It also stimulates macrophages to kill bacteria that have been engulfed. IFN-γ released by Th1 cells is also important in regulating the Th2 response. As IFN-γ is vitally implicated in the regulation of immune response, its production can lead to autoimmune disorders.
Viral induction of interferons
All classes of interferon are very important in fighting RNA virus infections. However, their presence also accounts for some of the host symptoms, such as sore muscles and fever. They are secreted when abnormally large amounts of dsRNA are found in a cell. dsRNA is normally present in very low quantities. The dsRNA acts like a trigger for the production of interferon. The gene that codes for this cytokine is switched on in an infected cell, and the interferon synthesized and secreted to surrounding cells.
As the original cell dies from the cytolytic RNA virus, these thousands of viruses will infect nearby cells. However, these cells have received interferon, which essentially warns these other cells that there's a wolf in the pack of sheep. They then start producing large amounts of a protein known as protein kinase R (or PKR). If a virus chooses to infect a cell that has been “pre-warned” by interferon, it is like charging into a hail of bullets for the virus. The PKR is activated by the dsRNA, and begins transferring phosphate groups (phosphorylating) to a protein known as eIF2, a eukaryotic translation initiation factor. After phosphorylation, eIF2 has a reduced ability to initiate translation, the production of proteins coded by cellular mRNA. This prevents viral replication, but also inhibits normal cell ribosome function, killing both the virus and the host cell if the response is active for a sufficient amount of time. All RNA within the cell is also degraded, preventing the mRNA from being translated by eIF2 if some of the eIF2 failed to be phosphorylated.
Pharmaceutical uses
Interferon was scarce and expensive until 1980 when the interferon gene was inserted into bacteria using recombinant DNA technology, allowing mass cultivation and purification from bacterial cultures. Several different types of interferon are now approved for use in humans, and interferon therapy is used (in combination with chemotherapy and radiation) as a treatment for many cancers. When used in the systemic therapy, IFN-α and IFN-γ are mostly administered by an intramuscular injection. The injection of interferons in the muscle, in the vein, or under skin is generally well tolerated. The most frequent side-effects are flu-like symptoms: increased body temperature, feeling ill, fatigue, headache, muscle pain, convulsion, dizziness, hair thinning, and depression. Erythema, pain and hardness on the spot of injection are also frequently observed. All known effects are usually reversible and disappear a few days after the therapy has been finished. However, there are some serious side effects and the patient is advised to read the accompanying pamphlet.
More than half of hepatitis C patients treated with interferon respond with better blood tests and better liver biopsies. There is some evidence that giving interferon immediately following infection can prevent hepatitis C; however, people infected by hepatitis C often do not display symptoms of HCV until months or years later.
More recently, the FDA approved pegylated interferon-alpha, in which polyethylene glycol is added to make the interferon last longer in the body. (Pegylated interferon-alpha-2b was approved in January 2001; pegylated interferon-alpha-2a was approved in October 2002.) The pegylated form is injected once weekly, rather than three times per week for conventional interferon-alpha. Used in combination with the antiviral drug ribavirin, pegylated interferon produces sustained cure rates of 75% or better in people with genotype 2 or 3 hepatitis C (which is easier to treat) but still less than 50% in people with genotype 1 (which is most common in the U.S. and Western Europe).
Interferon-beta (Interferon beta-1a and Interferon beta-1b) is used in the treatment and control of the neurological disorder multiple sclerosis. By an as-yet-unknown mechanism, interferon-beta inhibits the production of Th1 cytokines and the activation of monocytes.
Administered intranasally in very low doses, interferon is extensively used in Eastern Europe and Russia as a method to prevent and treat viral respiratory diseases such as cold and flu. It is claimed that the treatment can lower the risk of infection by as much as 60-70%. Mechanisms of such action of interferon are not well understood; it is thought that doses must be larger by several orders of magnitude to have any effect on the virus. Consequently, most Western scientists are sceptical of these claims.[19]
Also See
References
- ^ Nagano, Y. and Kojima,Y. (1954) “Pouvoir immunisant du virus vaccinal inactivé par des rayons ultraviolets” C.R. Seans. Soc. Biol.Fil 148:1700-1702
- ^ Nagano, Y. and Kojima,Y. (1958) “Pouvoir immunisant du virus vaccinal inactivé par des rayons ultraviolets” C.R. Seans. Soc. Biol.Fil 152:1672-1629
- ^ Wantanabe, Y. (2004) “Fifty Years of Interference”. Nature Immunology; 5(12):1193
- ^ Isaacs, A and Lindenmann J. 1957 "Virus Interference. I. The interferon" J. Proc. Roy. Soc. Lond. B Biol. Sci. 147;258-267
- ^ International Society For Interferon And Cytokine Research, October 2005 Volume 12, No. 3.
- ^ Oritani and Tomiyama, Interferon-ζ/limitin: Novel type I Interferon that displays a narrow range of biological activity. International journal of hematology, 2004, Volume 80, pages 325-331 .
- ^ Hardy et al., Characterization of the type I interferon locus and identification of novel genes. Genomics, 2004, Volume 84 pages 331-345.
- ^ Todd and Naylor, New chromosomal mapping assignments for argininosuccinate synthetase pseudogene 1, interferon-beta 3 gene, and the diazepam binding inhibitor gene. Somat. Cell. Mol. Genet. 1992 Volume 18, pages 381-5.
- ^ http://www.gene.ucl.ac.uk/nomenclature/data/get_data.php?hgnc_id=5452
- ^ http://www.gene.ucl.ac.uk/nomenclature/data/get_data.php?hgnc_id=5453
- ^ http://www.gene.ucl.ac.uk/nomenclature/data/get_data.php?hgnc_id=5454
- ^ http://www.gene.ucl.ac.uk/nomenclature/data/get_data.php?hgnc_id=5455
- ^ http://www.gene.ucl.ac.uk/nomenclature/data/get_data.php?hgnc_id=5449
- ^ http://www.gene.ucl.ac.uk/nomenclature/data/get_data.php?hgnc_id=5450
- ^ http://www.gene.ucl.ac.uk/nomenclature/data/get_data.php?hgnc_id=5451
- ^ Schultz et al., The interferon system of non-mammalian vertebrates. Developmental and Comparative Immunology, Volume 28, pages 499-508.
- ^ Vilcek, Novel interferons. Nature Immunology, 2003, Volume 4, pages 8-9
- ^ Bartlett et al., Murine interferon lambdas (type III interferons)exhibit potent antiviral activity in vivo in a poxvirus infection model. Journal of General Virology, 2005, Volume 86 pages 1589–1596
- ^ http://www.pathobiologics.org/ivphc/ref/iav121604.doc
Further reading
- Hall, Steven S. (1997) A Commotion in the Blood. New York, New York: Henry Holt and Company. ISBN 0-8050-5841-9
- Information on Interferon and how it relates to hepatitis c




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xxx
216.73.xxx.xxx
 Server Time:
Server Time: