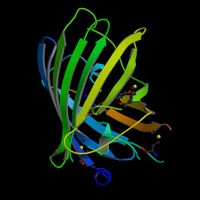
The green fluorescent protein (GFP) is a protein, comprised of 238 amino acids, from the jellyfish Aequorea victoria that fluoresces green when exposed to blue light. This process takes place when the protein aequorin, also produced by A. victoria, interacts with Ca2+ ions thus emitting a blue glow.
The wild-type GFP (wtGFP) from A. victoria has a major excitation peak at a wavelength of 395 nm and a minor one at 475 nm. Its emission peak is at 509 nm which is in the lower green portion of the visible spectrum. The GFP from the sea pansy (Renilla reniformis) has a single major excitation peak at 498 nm.
In cell and molecular biology, the GFP gene is frequently used as a reporter of expression. In modified forms it has been used to make biosensors, and many animals have been created that express GFP as a proof-of-concept that a gene can be expressed throughout a given organism.
One of the most powerful uses of GFP is to express the protein in small sets of specific cells. This allows researchers to optically detect specific types of cells in vitro (in a dish), or even in vivo (in the living organism). The GFP gene can be introduced into organisms and maintained in their genome through breeding, or local injection with a viral vector can be used to introduce the gene.
Due to this widespread usage different mutants of GFP have been engineered over the last few years by the lab of Roger Tsien and others: some mutants have been produced with increased fluorescence and the protein major excitation peak has been shifted to 490 nm with the peak emission kept at 509 nm (EGFP). Color mutants have been obtained from the GFP gene as well: in particular the cyan fluorescent protein (CFP) and the yellow fluorescent protein (YFP) are two colour variants employed for fluorescence resonance energy transfer (FRET) experiments. Genetically-encoded FRET reporters sensitive to cell signaling molecules, such as calcium or glutamate, protein phosphorylation state, protein complementation, receptor dimerization and other processes provide highly specific optical readouts of cell activity in real time.
EGFP is an enhanced green fluorescent protein which is also a commonly used gene reporter.
It is also worth mentioning that the availability of GFP and its derivatives has thoroughly redefined fluorescence microscopy and the way it is used in cell biology and other biological disciplines. While most small fluorescent molecules such as FITC (fluorescein isothiocyanate) are strongly phototoxic when used in live cells, fluorescent proteins such as GFP are usually much less harmful when illuminated in living cells. This has triggered the development of highly automated live cell fluorescence microscopy systems which can be used to observe cells over time expressing one or more proteins tagged with fluorescent proteins. Analysis of such time lapse movies has redefined the understanding of many biological processes which in the past had been studied using fixed (i.e. dead) material.
To date, many bacteria, yeast and other fungal cells, plant, fly, and mammalian cells have been created using GFP as a marker. Research scientists, from National Taiwan University's Department of Animal Science and Technology also reported the production of three fluorescent pigs in early 2006.
Notes
- Alba, a fluorescent bunny, was created by Eduardo Kac using GFP for purposes of art and social commentary [1].
External links
- Introduction to fluorescent proteins
- A discussion of the history, uses and structure of GFP hosted by Marc Zimmer a chemist at Connecticut College
- Lab website of Roger Tsien
- Interactive Java applet demonstating the chemistry behind the formation of the GFP fluorophore.




 216.73.216.81
216.73.216.81 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xx
216.73.xxx.xx
 Server Time:
Server Time: