| Glutamine | |
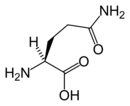  Chemical structure of L-glutamine |
|
| Systematic name | (2S)-2-amino-4-carbamoyl-butanoic acid |
| Chemical formula | C5H10N2O3 |
| Molar mass | 146.15 g mol−1 |
Glutamine (Gln) is one of the 20 amino acids encoded by the standard genetic code. Its side chain is an amide; it is formed by replacing a side-chain hydroxyl of glutamic acid with an amine functional group.
Contents |
Biochemistry
Formation and Nomenclature
Glutamine is genetically coded for by the RNA codons CAA and CAG. Glutamine's three-letter abbreviation is Gln, and its one-letter abbreviation is Q. A three-letter designation for either glutamine or glutamic acid is Glx (one-letter abbreviation: Z).
Like other amino acids, glutamine is biochemically important as a constituent of proteins. Glutamine is also crucial in nitrogen metabolism. Ammonia (formed by nitrogen fixation) is assimilated into organic compounds by converting glutamic acid to glutamine. The enzyme that accomplishes this is called glutamine synthetase. Glutamine can, hence, be used as a nitrogen donor in the biosynthesis of many compounds, including other amino acids, purines, and pyrimidines.
Nutrition
Occurrences in Nature
Glutamine is found in foods high in proteins, such as fish, red meat, beans, and dairy products.
Use
Glutamine is a supplement that is used in weightlifting, bodybuilding, endurance and other sports, as well as by those who suffer from muscular cramps or pain—particularly elderly people. It has been shown to speed up the Krebs Cycle, aiding in weight loss while retaining muscle[citation needed]. The main use of glutamine within the diet of either group is as a means of replenishing the body's stores of amino acids that have been used during exercise or everyday activities.
Studies which are looking into problems with excessive consumption of glutamine thus far have proved inconclusive. However, normal supplementation is healthy mainly because glutamine is supposed to be supplemented after prolonged periods of exercise (for example, a workout or exercise in which amino acids are required for use) and replenishes amino acid stores; this being the main reason glutamine is recommended during fasting or for people who suffer from physical trauma, immune deficiencies, or cancer.[1]
Aiding gastrointestinal function
There have been several recent studies into the effects of glutamine and what properties it possesses, and, there is now a significant body of evidence that links glutamine-enriched diets with intestinal effects; aiding maintenance of gut barrier function, intestinal cell proliferation and differentiation, as well as generally reducing septic morbidity and the symptoms of Irritable Bowel Syndrome. The reason for such "cleansing" properties is thought to stem from the fact that the intestinal extraction rate of glutamine is higher than that for other amino acids, and is therefore thought to be the most viable option when attempting to alleviate conditions relating to the gut. [1]
These conditions were discovered after comparing plasma concentration within the gut between glutamine-enriched and non glutamine-enriched diets. However, even though glutamine is thought to have "cleansing" properties and effects, it is unknown to what extent glutamine has clinical benefits, due to the varied concentrations of glutamine in varieties of food. [1]
Aiding recovery after surgery
It is also known that glutamine has various effects in reducing healing time after operations. Hospital waiting times after abdominal surgery are reduced by providing parenteral nutrition regimens containing amounts of glutamine to patients. Clinical trials have revealed that patients on supplementation regimes containing glutamine have improved nitrogen balances, generation of cysteinyl-leukotrienes from polymorphonuclear neutrophil granulocytes and improved lymphocyte recovery and intestinal permeability (in postoperative patients) - in comparison to those who had no glutamine within their dietary regime; all without any side-effects. [3]
References
- ^ Glutamine used for the Immune System and Cancer. Retrieved on 2006-07-28.
- a
b
 Boza J.J., Dangin M., Moennoz D., Montigon F., Vuichoud
J., Jarret A., Pouteau E., Gremaud G., Oguey-Araymon S.,
Courtois D., Woupeyi A., Finot P.A. and Ballevre O. Free
and protein-bound glutamine have identical splanchnic
extraction in healthy human volunteers. Am J Physiol
Gastrointest Liver Physiol. 2001 Jul; 281(1): G267-74.
PMID 11408280
Free text
Boza J.J., Dangin M., Moennoz D., Montigon F., Vuichoud
J., Jarret A., Pouteau E., Gremaud G., Oguey-Araymon S.,
Courtois D., Woupeyi A., Finot P.A. and Ballevre O. Free
and protein-bound glutamine have identical splanchnic
extraction in healthy human volunteers. Am J Physiol
Gastrointest Liver Physiol. 2001 Jul; 281(1): G267-74.
PMID 11408280
Free text
- a McAnena O.J., Moore F.A., Moore E.E., Jones T.N. and Parsons P. Selective uptake of glutamine in the gastrointestinal tract: confirmation in a human study. Br J Surg. 1991 Apr; 78(4): 480-2. PMID 1903318
- a Morlion B.J., Stehle P., Wachtler P., Siedhoff H.P., Koller M., Konig W., Furst P., Puchstein C. Total parenteral nutrition with glutamine dipeptide after major abdominal surgery. Ann Surg. 1998 Feb; 227(2): 302-8. PMID 9488531
- a Jiang Z.M., Cao J.D., Zhu X.G., Zhao W.X., Yu J.C., Ma E.L., Wang X.R., Zhu M.W., Shu H., Liu Y.W. The impact of alanyl-glutamine on clinical safety, nitrogen balance, intestinal permeability, and clinical outcome in postoperative patients: a randomised, double-blind, controlled study of 120 patients. JPEN J Parenter Enteral Nutr. 1999 Sep-Oct;23(5 Suppl):S62-6. PMID 10483898
External links
- Computational Chemistry Wiki
- Nutrients and HIV: Part Three
- Link page to external chemical sources.
- Supplemental uses




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xxx
216.73.xxx.xxx
 Server Time:
Server Time: