 |
|
|
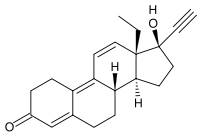
Gestrinone
|
|
| Systematic (IUPAC) name | |
| 13-ethyl-17alpha-hydroxy-18,19 dinorpregna-4,9,11-trien-20-yn-3-one | |
| Identifiers | |
| CAS number | 16320-04-0 |
| ATC code | G03XA02 |
| PubChem | 27812 |
| Chemical data | |
| Formula | C21H24O2 |
| Mol. weight | 308.4 g/mol |
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Excretion | Renal, fecal |
| Therapeutic considerations | |
| Pregnancy cat. | X |
Gestrinone is a synthetic steroid hormone that acts as an anti-progestin and also has some androgenic activity. It is marketed under the names Dimetriose, Dimetrose, and Nemestran, as a treatment for endometriosis. Gestrinone is available in many countries, but not in the USA. As it has anabolic effects, its use in competition has been banned by the International Olympic Committee.[1]
Contents |
Method of action
Its mechanism of action consists of suppression of the release of pituitary gonadotropins. Gestrinone also interacts with the endometrium, inhibiting its growth. The inhibition is the result of gestrinone's interaction with the androgen receptor; this is also the reason for androgenic side effects. Gestrinone has been shown to interact with the estrogen receptor, the androgen receptor, and the progesterone receptor.[2]
Metabolism
The drug is well absorbed via the oral route, passed through the liver, and has a half-life of about 24 hours. It is metabolized by the liver and excreted by urine and feces.
Contraindications and side effects
The drug is contraindicated in pregnancy, during lactation, and in patients with severe cardiac, renal or hepatic insufficiency. It is also contraindicated in patients who experienced metabolic and/or vascular disorders during previous estrogen or progestogen therapy, or who are allergic to the medication. The drug is contraindicated in children.
Side effects include vaginal spotting, and, in susceptible individuals, signs of increased androgen activity such as acne, oily skin, fluid retention, weight gain, hirsutism, voice change, or hair loss.
Other uses
The drug has also been investigated for use as a prospective contraceptive agent and as a postcoital contraceptive.[3] It also has been used to shrink uterine fibroids and to reduce menorrhagia[4][5]
Its androgenic properties are more exploited in a "designer steroid", the derivative tetrahydrogestrinone. THG was banned by the Food and Drug Administration (FDA) in 2003.
References
- ^ Helping athletes compete drug-free (PDF) pp. 34. Canadian Centre for Ethics in Sport (May 2000). Retrieved on 2006-06-01.
- ^ Tamaya, T., Fujimoto J., Watanabe Y., Arahori K. & Okada H. (1986). "Gestrinone (R2323) binding to steroid receptors in human uterine endometrial cytosol". Acta obstetricia et gynecologica Scandinavica 65 (5): 439-41. PMID 3490730. Retrieved on 2006-06-01.
- ^ Emergency Contraception Update (RTF) pp. 5. International Consortium for Emergency Contraception (October 2006). Retrieved on 2006-06-01.
- ^ La Marca, A., Giulini S., Vito G., Orvieto R., Volpe A. & Jasonni V.M. (December 2004). "Gestrinone in the treatment of uterine leiomyomata: effects on uterine blood supply". Fertility and Sterility 82 (6): 1694-6. PMID 15589885. Retrieved on 2006-06-01.
- ^ Roy, SN., Bhattacharya S. (2004). "Benefits and risks of pharmacological agents used for the treatment of menorrhagia". Drug safety : an international journal of medical toxicology and drug experience 27 (2): 75-90. PMID 14717620. Retrieved on 2006-06-01.




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xxx
216.73.xxx.xxx
 Server Time:
Server Time: