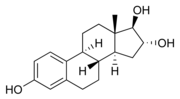
 Estriol. Note two hydroxyl (-OH) groups attached to the D ring
(rightmost ring).
Estriol. Note two hydroxyl (-OH) groups attached to the D ring
(rightmost ring).
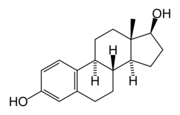 Estradiol. Note one
hydroxyl group attached to the D ring. The 'di'
refers both to this hydroxyl and the one on the
A ring (leftmost).
Estradiol. Note one
hydroxyl group attached to the D ring. The 'di'
refers both to this hydroxyl and the one on the
A ring (leftmost).
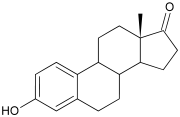 Estrone. Note the ketone (=O) group attached to the D ring.
Estrone. Note the ketone (=O) group attached to the D ring.
Estrogens (also oestrogens) are a group of steroid compounds, named for their importance in the oestrus cycle, and functioning as the primary female sex hormone. While estrogens are present in both men and women, they are usually present at significantly higher levels in women of reproductive age. They promote the development of female secondary sex characteristics, such as breasts, and are also involved in the thickening of the endometrium and other aspects of regulating the menstrual cycle.
Contents |
Explanation
The three major naturally occurring estrogens in women are estradiol, estriol and estrone. From menarche to menopause the primary estrogen is estradiol 17beta. In the body these are all produced from androgens through enzyme action. Estradiol is produced from testosterone and estrone from androstenedione. Estrone is weaker than estradiol, and in post-menopausal women more estrone is present than estradiol.
Estrogens are used as part of some oral contraceptives and also in estrogen replacement therapy of post-menopausal women.
Like all steroid hormones, estrogens readily diffuse across the cell membrane. Inside the cell, they interact with estrogen receptors.[1] A range of synthetic and natural substances have been identified that also possess estrogenic activity.[2] Synthetic substances of this kind are known as xenoestrogens, while natural plant products with estrogenic activity are called phytoestrogens.
Estrogen production
Estrogen is produced primarily by developing follicles in the ovaries, the corpus luteum and the placenta. Follicle stimulating hormone (FSH) and luteinizing hormone (LH) stimulate the production of estrogen in the ovaries. Some estrogens are also produced in smaller amounts by other tissues such as the liver, adrenal glands and the breasts. These secondary sources of estrogen are especially important in post-menopausal women.
Synthesis of estrogens starts in theca interna cells in the ovary, by the synthesis of androstenedione from cholesterol. Androstenedione is a substance of moderate androgenic activity. This compound crosses the basal membrane into the surrounding granulosa cells, where it is converted to estrone or estradiol, either immediately or through testosterone. The conversion of testosterone to estradiol, and of androstenedione to estrone, is catalyzed by the enzyme aromatase.
Estradiol levels vary through the menstrual cycle, with levels highest just before ovulation.
Functions of estrogens
- structural
- promote formation of female secondary sex characteristics
- stimulate endometrial growth
- increase uterine growth
- maintenance of vessel and skin
- reduce bone resorption, increase bone formation
-
protein synthesis
- increase hepatic production of binding proteins
-
coagulation
- increase circulating level of factors 2,7,9,10, antithrombin III, plasminogen
- increase platelet adhesiveness
- lipid
- increase HDL, triglyceride, fat deposit
- decrease LDL
- fluid balance
- salt and water retention
- gastro-intestinal tract
- reduce bowel motility
- increase cholesterol in bile
Studies have found better correlation between sexual desire and androgen levels than for estrogen levels.[3]
In studies involving mice and rats, it was found that lung function may be improved by estrogen. In one study involving 16 animals, female mice that had their ovaries removed to deprive them of estrogen lost 45 percent of their working alveoli from their lungs. Upon receiving estrogen, the mice recovered full lung function. Two proteins that are activated by estrogen play distinct roles in breathing. One protein builds new alveoli, the other stimulates the alveoli to expel carbon dioxide. Loss of estrogen hampered both functions in the test mice.[4]
Medical applications
Since estrogen circulating in the blood can feedback to reduce circulating levels of FSH and LH, most oral contraceptives contain a synthetic estrogen, along with a synthetic progestin.
In hormone replacement therapy, estrogen and other hormones are given to postmenopausal women in order to prevent osteoporosis as well as treat the symptoms of menopause such as hot flashes, vaginal dryness, urinary stress incontinence, chilly sensations, dizziness, fatigue, irritability, and sweating. Fractures of the spine, wrist, and hips decrease by 50-70% and spinal bone density increases by ~5% in those women treated with estrogen within 3 years of the onset of menopause and for 5-10 years thereafter. Standard therapy is 0.625 mg/day of conjugated estrogens (such as is in Premarin), but the dose can range from 0.3 mg/day to 1.25 mg/day. Estrogen replacement therapy also has favorable effects on serum cholesterol levels and is claimed to dramatically reduce the incidence of cardiovascular disease. There are, however, risks associated with estrogen therapy. Among the older postmenopausal women studied as part of the Women's Health Initiative (WHI), an orally-administered estrogen supplement has been associated with an increased risk of dangerous blood clotting. The WHI studies used one type of estrogen supplement, a high oral dose of conjugated equine estrogens (Premarin alone and with Provera as Prempro)[1]. Research is underway to determine if risks of estrogen supplement use are the same for all estrogen supplement types. In particular, topically-applied estrogen may have a different spectrum of side-effects than does estrogen administered by the oral route[2].
Estrogen is also used in the therapy of vaginal atrophy, hypoestrogenism (as a result of hypogonadism, castration, or primary ovarian failure), amenorrhea, dysmenorrhea, and oligomenorrhea. Estrogens can also be used to suppress lactation after child birth.
Health risks and warning labels
The labeling of estrogen-only products includes a boxed warning that unopposed estrogen (without progestogen) therapy increases the risk of endometrial cancer.
Based on a review of data from the WHI, on January 8, 2003 the FDA changed the labeling of all estrogen and estrogen with progestin products for use by postmenopausal women to include a new boxed warning about cardiovascular and other risks. The estrogen-alone substudy of the WHI reported an increased risk of stroke and deep vein thrombosis (DVT) in postmenopausal women 50 years of age or older and an increased risk of dementia in postmenopausal women 65 years of age or older using 0.625 mg of Premarin conjugated equine estrogens (CEE). The estrogen-plus-progestin substudy of the WHI reported an increased risk of myocardial infarction, stroke, invasive breast cancer, pulmonary emboli and DVT in postmenopausal women 50 years of age or older and an increased risk of dementia in postmenopausal women 65 years of age or older using 0.625 mg of CEE with 2.5 mg of the progestin medroxyprogesterone acetate (MPA).[5][6][7]
Estrogens in cosmetics
Some hair shampoos on the market include estrogens and placental extracts; others contain phytoestrogens. There are case reports of young children developing breasts after exposure to these shampoos. The FDA is aware of these reports but does not consider them of concern to consumers.[8] These products are especially popular with African-American consumers.[9][10]
On September 9, 1993, the FDA determined that all topically-applied hormone-containing drug products for OTC human use are not generally recognized as safe and effective and are misbranded. An accompanying proposed rule deals with cosmetics, concluding that any use of natural estrogens in a cosmetic product makes the product an unapproved new drug and that any cosmetic using the term "hormone" in the text of its labeling or in its ingredient statement makes an implied drug claim, subjecting such a product to regulatory action.[11]
In addition to being considered misbranded drugs, products claiming to contain placental extract may also be deemed to be misbranded cosmetics if the extract has been prepared from placentas from which the hormones and other biologically active substances have been removed and the extracted substance consists principally of protein. The FDA recommends that this substance be identified by a name other than "placental extract" and describing its composition more accurately because consumers associate the name "placental extract" with a therapeutic use of some biological activity.[11]
The History of Estrogen
The existence and effects of estrogen were established from 1923 to 1938 in which the formulation was led by a group of scientists instead of pharmaceutical companies. Thereafter, the market for hormonal drug research opened up.
The “first orally effective estrogen”, Emmenin, derived from the late-pregnancy urine of Canadian women was introduced in 1930 by Collip and Ayerst Laboratories. However, scientists continued to search for new sources of estrogen because of the concerns associated with the practicality of introducing the drug into the market. At the same time, a German pharmaceutical drug company, Schering, formulated a similar product as Emmenin that was introduced to German women to treat menopausal symptoms.
In 1938, British scientists obtained a patent on a newly formulated nonsteroidal estrogen, Diethylstilbestrol (DES), that was cheaper and more powerful than the previously manufactured estrogens. Soon after, concerns over the side effects of DES were raised in scientific journals while the drug manufacturers came together to lobby for governmental approval of DES. It was only until 1941 when estrogen therapy was finally approved by the Food and Drug Administration (FDA) for the treatment of menopausal symptoms. [12]
References
- ^ Nussey and Whitehead: Endocrinology, an integrated approach, Taylor and Francis 2001
- ^ Fang H, Tong W, Shi LM, Blair R, Perkins R, Branham W, Hass BS, Xie Q, Dial SL, Moland CL, Sheehan DM. Structure-activity relationships for a large diverse set of natural, synthetic, and environmental estrogens. Chemical Research in Toxicology 2001;14:280-294. PMID 11258977.
- ^ Warnock JK, Swanson SG, Borel RW, Zipfel LM, Brennan JJ. (2005). "Combined esterified estrogens and methyltestosterone versus esterified estrogens alone in the treatment of loss of sexual interest in surgically menopausal women". Menopause 12 (4): 374-84. PMID 16037752.
- ^ Massaro D, Massaro GD (2004). "Estrogen regulates pulmonary alveolar formation, loss, and regeneration in mice". American Journal of Physiology. Lung Cellular and Molecular Physiology 287 (6): L1154-9. PMID 15298854.
- ^ FDA (2003, Jan 8). FDA Approves New Labels for Estrogen and Estrogen with Progestin Therapies for Postmenopausal Women Following Review of Women's Health Initiative Data. Retrieved on 2006-10-26.
- ^ Kolata, Gina (2003, Jan 9). F.D.A. Orders Warning on All Estrogen Labels. The New York Times. Retrieved on 2006-10-26.
- ^ NLM (2006, Apr 1). IMPORTANT WARNING. Drug Information: Estrogen. MedlinePlus. Retrieved on 2006-10-26.
- ^ Preschool Puberty, and a Search for the Causes, The New York Times, 17 October 2006.
- ^ Shampoos Contain Large Clinical Doses of Estrogen that Will Cause Breast Cysts, New Scientist, April 03, 2002
- ^ Su-Ting et al. Hormone-Containing Hair Product Use in Prepubertal Children. Pediatrics & Adolescent Medicine, vol 156, p. 85. January 2002. PMID 11772198
- ^ a b FDA (1995, Feb). Products containing estrogenic hormones, placental extract or vitamins. Guide to Inspections of Cosmetic Product Manufacturers. Retrieved on 2006-10-24.
- ^ Rothenberg, Carla J. (2005-04-25). The Rise and Fall of Estrogen Therapy: The History of HRT. Retrieved on 2006-10-27.
External links and further reading
- MedlinePlus Drug Information: Estrogen information on estrogen-only prescription drugs from the U.S. National Library of Medicine
- Nussey and Whitehead: Endocrinology, an integrated approach, Taylor and Francis 2001. Free online textbook.




 216.73.216.81
216.73.216.81 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xx
216.73.xxx.xx
 Server Time:
Server Time: