 |
|
|
Erythromycin
|
|
| Systematic (IUPAC) name | |
|
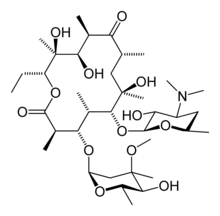
6-(4-dimethylamino-3-hydroxy-6-methyl-oxan-2-yl)oxy-14-ethyl-7,12,13-trihydroxy-
4-(5-hydroxy-4-methoxy-4,6-dimethyl-oxan-2-yl)oxy-3,5,7,9,11,13-hexamethyl- 1-oxacyclotetradecane-2,10-dione |
|
| Identifiers | |
| CAS number | 114-07-8 |
| ATC code | J01FA01 |
| PubChem | 3255 |
| DrugBank | APRD00953 |
| Chemical data | |
| Formula | C37H67NO13 |
| Mol. weight | 733.93 g/mol |
| Pharmacokinetic data | |
| Bioavailability | 100% |
| Protein binding | 90% |
| Metabolism | liver (under 5% excreted unchanged) |
| Half life | 1.5 hours |
| Excretion | bile |
| Therapeutic considerations | |
| Pregnancy cat. | A(AU) B(US) |
| Legal status | ℞ Prescription only |
| Routes | oral, iv, im |
Erythromycin (also known as eryth ethylsuc) is a macrolide antibiotic which has an antimicrobial spectrum similar to or slightly wider than that of penicillin, and is often used for people who have an allergy to penicillins. For respiratory tract infections, it has better coverage of atypical organisms, including mycoplasma. It is also used to treat outbreaks of chlamydia, syphilis, acne and gonorrhea. Structurally, this macrocyclic compound contains a 14-membered lactone ring with ten asymmetric centers and two sugars (L-cladinose and D-desoamine), making it a compound very difficult to produce via synthetic methods.
Erythromycin is produced from a strain of the actinomyces Saccaropolyspora erythraea, formerly known as Streptomyces erythraeus.
Contents |
History
Abelardo Aguilar, a Filipino scientist, sent some soil samples to his employer Eli Lilly in 1949. Eli Lilly’s research team, led by J. M. McGuire, managed to isolate Erythromycin from the metabolic products of a strain of Streptomyces erythreus (designation changed to "Saccharopolyspora erythraea") found in the samples. Lilly filed for patent protection of the compound and U.S. patent 2,653,899 was granted in 1953. The product was launched commercially in 1952 under the brand name Ilosone® (after the Philippine region of Iloilo where it was originally collected from). Erythromycin was formerly also called Ilotycin®. In 1981, Nobel laurate (1965 in chemistry) and Professor of Chemistry at Harvard University (Cambridge, MA) Robert B. Woodward and a large team of researchers reported the first stereocontrolled asymmetric chemical synthesis of Erythromycin A.
The antiobiotic clarithromycin was invented by scientists at the Japanese drug company Taisho Pharmaceutical in the 1970s as a result of their efforts to overcome the acid instability of erythromycin.
Available forms
Erythromycin is available in enteric-coated tablets, slow release capsules, oral suspensions, ophthalmic solutions, ointments, gels and injections.
Brand names include Robimycin, E-Mycin, E.E.S. Granules, E.E.S.-200, E.E.S.-400, E.E.S.-400 Filmtab, Erymax, Ery-Tab, Eryc, Erypar, EryPed, Eryped 200, Eryped 400, Erythrocin Stearate Filmtab, Erythrocot, E-Base, Ilosone, MY-E, Pediamycin, Zineryt and PCE Dispertab.
Mechanism of action
Erythromycin prevents bacteria from growing by interfering with their protein synthesis. Erythromycin binds to the 23s rRNA molecule in the 50S of the bacterial ribosome, blocking the exit of the growing peptide chain thus inhibiting the translocation of peptides.
Pharmacokinetics
Erythromycin is easily inactivated by gastric acids, therefore all orally administered formulations are given as either enteric coated or as more stable salts or esters. Erythromycin is very rapidly absorbed, and diffused into most tissues and phagocytes. Due to the high concentration in phagocytes, erythromycin is actively transported to the site of infection, where during active phagocytosis, large concentrations of erythromycin are released.
Metabolism
Most of erythromycin is metabolised by demethylation in the liver. Its main elimination route is in the bile, and a small portion in the urine. Erythromycin's half-life is 1.5 hours.
Side-effects
Gastrointestinal disturbances such as diarrhea, nausea, abdominal pain and vomiting are fairly common so it tends not to be prescribed as a first-line drug. However, erythromycin may be useful in treating gastroparesis due to this pro-motility effect. Intravenous erythromycin may also be used in endoscopy as an adjunct to clear gastric contents.
More serious side-effects, such as reversible deafness are rare. Allergic reactions, while uncommon, may occur, ranging from urticaria to anaphylaxis. Cholestasis, Stevens-Johnson syndrome and toxic epidermal necrolysis are some other rare side effects that may occur.
Erythromycin has been shown to increase the probability of pyloric stenosis in children whose mothers took the drug during the late stages of pregnancy or while nursing.
Contraindications
Earlier case reports on sudden death prompted a study on a large cohort that confirmed a link between erythromycin, ventricular tachycardia and sudden cardiac death in patients also taking drugs that prolong the metabolism of erythromycin (like verapamil or diltiazem) by interfering with CYP3A4 (Ray et al 2004). Hence, erythromycin should not be administered in patients using these drugs, or drugs that also prolong the QT time. Other examples include terfenadine (Seldane, Seldane-D), astemizole (Hismanal), cisapride (Propulsid, withdrawn in many countries for prolonging the QT time) and pimozide (Orap).
Some people can be allergic to Erythromycin and a small dose can cause massive liver failure.
References
- Ray WA, Murray KT, Meredith S, Narasimhulu SS, Hall K, Stein CM. Oral Erythromycin and the Risk of Sudden Death from Cardiac Causes. N Engl J Med 2004;351:1089-96.
- British National Formulary "BNF 49" March 2005.




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xxx
216.73.xxx.xxx
 Server Time:
Server Time: