 |
|
|
Dehydroepiandrosterone
|
|
| Systematic (IUPAC) name | |
| 3ß-hydroxy-5-androsten-17-one | |
| Identifiers | |
| CAS number | 53-43-0 |
| ATC code | A14AA07 |
| PubChem | 76 |
| Chemical data | |
| Formula | C19H28O2 |
| Mol. weight | 288.43 |
| Physical data | |
| Melt. point | 148.5 °C (299 °F) |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | Hepatic |
| Half life | 12 hours |
| Excretion | Urinary:?% |
| Therapeutic considerations | |
| Legal status | Commercially available (US) |
| Routes | Oral |
Dehydroepiandrosterone (DHEA), is a natural steroid hormone produced from cholesterol by the adrenal glands, the gonads, adipose tissue and the brain. DHEA is the precursor of, androstenedione, testosterone and estrogen. It is the most abundant hormone in the human body.
Contents |
Synonyms and brand names
Synonyms for Dehydroepiandrosterone are: Dehydroisoandrosterone; 3β-Hydroxy-5-androsten-17-one; 3β-Hydroxyandrost-5-en-17-one; Androstenol; Androstenolone; Dehydroisoandrosterone; Hydroxyandrost-5-en-17-one; Prasterone; trans-Dehydroandrosterone.
Brand names for DHEA include Prastera® and Fidelin®.
DHEAS (Dehydroepiandrosterone sulfate)
Dehydroepiandrosterone sulfate (DHEAS, PubChem 12594) is the sulfated version of DHEA, - this conversion is reversibly catalyzed by sulfotransferase (SULT2A1) primarily in the adrenals, the liver, and small intestines. In blood, most DHEA is found as DHEAS with levels that are about 300 times higher than free DHEA. Orally ingested DHEA is converted to its sulfate when passing through intestines and liver. While DHEA levels reach their peak in the early morning hours, DHEAS levels show no diurnal variation.
From a practical point measurement of DHEAS is preferable to DHEA as levels are more stable.
Production
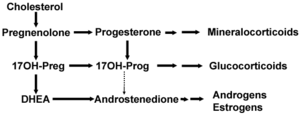
DHEA is produced from cholesterol through two cytochrome P450 enzymes. Cholesterol is converted to pregnenolone by the enzyme P450 scc (side chain cleavage) and then another enzyme CYP17A1 converts pregnenolone to 17α-Hydroxypregnenolone and then to DHEA. In humans DHEA is the dominant steroid hormone and precursor of all sex steroids. Humans produce DHEA in greater quantity than any other species. Even non-human primates have not much more than 10% the relative serum level of DHEA seen in humans. The fact that rodents produce so little DHEA makes the results of experiments conducted with these laboratory animals very controversial.
DHEA production is very high during fetal life by the fetal adrenal glands, declines after birth and remains low during childhood. Production begins around 6 years of age, increasing in quantity until peaking in early adulthood, around the age of 25, and declines afterwards to approximately 10% of peak levels by age 80. It is theorized by some that this decline may be due to reduced oxygen and glucose supply to the adrenal glands as a result of age-related atherosclerosis.
Role
In a simple view DHEA can be understood as a prohormone for the sex steroids. Its DHEAS variation may be looked at as buffer and reservoir. Its production in the brain suggests that is also has a role as a neurosteroid. As most DHEA is produced by the zona reticularis of the adrenal, it is argued that there is a role in the immune and stress response. DHEA may have more biologic roles.
As almost all DHEA is derived from the adrenal glands, blood measurements of DHEAS/DHEA are useful to detect excess adrenal activity as seen in adrenal cancer or hyperplasia, including certain forms of congenital adrenal hyperplasia. Women with polycystic ovary syndrome tend to have normal or mildly elevated levels of DHEAS.
Effects
Studies have shown that DHEA is useful in patients with systemic lupus erythematosus. An application of the evidence was reviewed by the FDA in 2001 and is available online.[1] This review also shows that cholesterol and other serum lipids decrease with the use of DHEA.
Supplementation with DHEA has been shown to decrease insulin resistance.[2]
Long term supplementation has been shown to improve mood and relieve depression.[3]
Disputed effects
The significance of the hormone in health and disease is not fully established. It is postulated that DHEA supplements are beneficial in alleviating:
- cardiovascular disease
diabetes
hypercholesterolemia
obesity
multiple sclerosis - Parkinson's disease
- Alzheimer's disease
disorders of the immune system
depression
osteoporosis
decreased libido
decreased orgasmic intensity
It is also commercially advertised that DHEA:
- helps decrease insulin resistance
- improves fat metabolism
- increases immune system function
- has anti-aging properties
- increases lean muscle mass
7-Keto™ DHEA, a recently identified natural metabolite of dehydroepiandrosterone (DHEA) is claimed to be both more effective and safer than DHEA because it does not convert itself into testosterone or estrogens in the body.
DHEA and DHEAS are readily available in the United States, but not in many other countries.
Precautions
Some assert that DHEA should not be supplemented outside specialist centres under careful observation of experts in the field of endocrinology.
Side effects may include:
- Palpitations and other arrhythmias
extensive growth of body hair, or hirsutism
Hair loss, especially male pattern baldness
acne
Contraindication
As DHEAS and DHEA are converted to sex steroids, their use is contraindicated in patients with any cancer that is estrogen or testosterone dependent.
Increasing endogenous production
Regular exercise is known to increase DHEA production in the body.[4][5][6] Caloric restriction has also been shown to increase DHEA in primates.[7]
References
- ^ FDA document regading DHEA and SLE
- ^ Kawano H, Yasue H, Kitagawa A, et al. Dehydroepiandrosterone supplementation improves endothelial function and insulin sensitivity in men. J Clin Endocrinol Metab. 2003 Jul;88(7):3190-5.
- ^ Wolkowitz OM, Reus VI, Roberts E, et al. Antidepressant and cognition-enhancing effects of DHEA in major depression. Ann NY Acad Sci. 1995 Dec 29;774:337-9
- ^ Eur J Appl Physiol Occup Physiol 1998 Oct;78(5):466-71
- ^ Eur J Appl Physiol. 2001 Jul;85(1- 2):177-84
- ^ J Gerontol A Biol Sci Med Sci. 2002 Apr;57(4):B158-65
- ^ Exp Gerontol. 2003 Jan-Feb; 38(1-2):35-46
Further reading
- Nutrition through the Life Cycle, Judith E. Brown, ISBN 0-534-58986-3




 216.73.216.81
216.73.216.81 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xx
216.73.xxx.xx
 Server Time:
Server Time: