 |
|
|
Clopidogrel
|
|
| Systematic (IUPAC) name | |
|
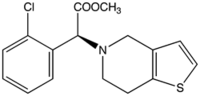
(+)-(S)-methyl
2-(2-chlorophenyl)- 2-(6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl)acetate |
|
| Identifiers | |
| CAS number | 113665-84-2 |
| ATC code | B01AC04 |
| PubChem | 60606 |
| DrugBank | APRD00444 |
| Chemical data | |
| Formula | C16H16ClNO2S |
| Mol. weight | 321.82 |
| Pharmacokinetic data | |
| Bioavailability | >50% |
| Metabolism | hepatic |
| Half life | 7–8 hours (inactive metabolite) |
| Excretion | 50%
renal 46% biliary |
| Therapeutic considerations | |
| Pregnancy cat. | B1(AU) B(US) |
| Legal status | S4(AU) POM(UK) ℞-only(US) |
| Routes | oral |
Clopidogrel is a potent oral antiplatelet agent often used in the treatment of coronary artery disease, peripheral vascular disease, and cerebrovascular disease. It is marketed by Bristol-Myers Squibb and Sanofi-Aventis under the trade name Plavix. It is also marketed in the generic form by Apotex, a Canadian generic pharmaceutical company, though an injunction to withhold further shipments of their form is in effect while patent issues are dealt with. In 2005 it was the world's second highest selling pharmaceutical with sales of US$5.9 billion. [1]
Contents |
Pharmacology
The mechanism of action of clopidogrel is irreversible blockade of the adenosine diphosphate (ADP) receptor on platelet cell membranes. This receptor is named P2Y12 and is important in platelet aggregation, the cross-linking of platelets by fibrin. The blockade of this receptor inhibits platelet aggregation by blocking activation of the glycoprotein IIb/IIIa pathway.
Platelet inhibition can be demonstrated two hours after a single dose of oral clopidogrel.
Clinical use
Indications
Clopidogrel is indicated for (Rossi, 2006):
- Prevention of vascular ischaemic events in patients with symptomatic atherosclerosis
- Acute coronary syndrome without ST-segment elevation (NSTEMI), along with aspirin
It is also used, along with aspirin, for the prevention of thromboembolism after placement of intracoronary stent. (Rossi, 2006)
Most consensus-based therapeutic guidelines recommend the use of clopidogrel, over aspirin, in patients requiring antiplatelet therapy but with a history of gastric ulceration, due to the lower incidence of gastric ulceration associated with the use of clopidogrel vs aspirin. A recent study has shown that in patients with healed aspirin-induced ulcers, however, patients receiving aspirin plus the proton pump inhibitor esomeprazole had a lower incidence of recurrent ulcer bleeding than patients receiving clopidogrel. (Chan et al., 2005)
Dosage forms
Clopidogrel is marketed as clopidogrel bisulfate (clopidogrel hydrogen sulfate), most commonly under the trade name Plavix, as 75 mg tablets.
Adverse effects
Serious adverse drug reactions associated with clopidogrel therapy include:
- Severe neutropenia (Incidence: 5/10,000)
- Thrombotic thrombocytopenic purpura (TTP) (Incidence: 4/1,000,000 patients treated)
-
Hemorrhage - The incidence of hemorrhage may be
increased by the co-administration of
aspirin.
- Gastrointestinal Hemorrhage (Incidence: 2.0%)
- Cerebral Hemorrhage (Incidence: 0.1 to 0.4%)
References
- Chan FKL, Ching JYL, Hung LCT, Wong VWS, Leung VKS, Kung NNS, et al. Clopidogrel versus aspirin and esomeprazole to prevent recurrent ulcer bleeding. New Engl J Med 2005;352(3):238-244.
- Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006. ISBN 0-9757919-2-3




 216.73.216.133
216.73.216.133 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xxx
216.73.xxx.xxx
 Server Time:
Server Time: