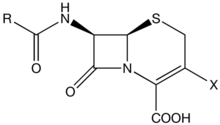
The cephalosporins (IPA: [ˌkɛfəloˈspɔrən, ˌsɛfə-]) are a class of β-lactam antibiotics. Together with cephamycins they belong to a sub-group called cephems.
Contents |
History
Cephalosporin compounds were first isolated from cultures of Cephalosporium acremonium from a sewer in Sardinia in 1948 by Italian scientist Giuseppe Brotzu. He noticed that these cultures produced substances that were effective against Salmonella typhi, the cause of typhoid fever. Researchers at the Sir William Dunn School of Pathology at the University of Oxford isolated cephalosporin C, which had stability to β-lactamases but was not sufficiently potent for clinical use. The cephalosporin nucleus, 7-aminocephalosporanic acid (7-ACA), was derived from cephalosporin C and proved to be analogous to the penicillin nucleus 6-aminopenicillanic acid. Modification of the 7-ACA side-chains resulted in the development of useful antibiotic agents, and the first agent cephalothin (cefalotin) was launched by Eli Lilly in 1964.
Mode of action
Cephalosporins are bactericidal and have the same mode of action as other beta-lactam antibiotics (such as penicillins). Cephalosporins disrupt the synthesis of the peptidoglycan layer of bacterial cell walls. The peptidoglycan layer is important for cell wall structural integrity, especially in Gram-positive organisms. The final transpeptidation step in the synthesis of the peptidoglycan is facilitated by transpeptidases known as penicillin binding proteins (PBPs).
Clinical use
Cephalosporins are indicated for the prophylaxis and treatment of bacterial infections caused by susceptible organisms. First-generation cephalosporins are predominantly active against Gram-positive bacteria, and successive generations have increased activity against Gram-negative bacteria (albeit often with reduced activity against Gram-positive organisms).
Adverse effects
Common adverse drug reactions (ADRs) (≥1% of patients) associated with cephalosporin therapy include: diarrhea, nausea, rash, electrolyte disturbances, and/or pain and inflammation at injection site. Infrequent ADRs (0.1–1% of patients) include: vomiting, headache, dizziness, oral and vaginal candidiasis, pseudomembranous colitis, superinfection, eosinophilia, and/or fever.
Approximately 5–10% of patients with allergic hypersensitivity to penicillins and/or carbapenems will also have cross-reactivity with cephalosporins. Thus, they are contraindicated in patients with a history of severe of immediate allergic reactions (urticaria, anaphylaxis, interstitial nephritis, etc) to penicillins, carbapenems or cephalosporins.[1]
Classification
The cephalosporin nucleus can be modified to gain different properties. Cephalosporins are sometimes grouped into "generations" by their antimicrobial properties. The first cephalosporins were designated first generation while later, more extended spectrum cephalosporins were classified as second generation cephalosporins. Each newer generation of cephalosporins has significantly greater Gram-negative antimicrobial properties than the preceding generation, in most cases with decreased activity against Gram-positive organisms. Fourth generation cephalosporins, however, have true broad spectrum activity.
The classification of cephalosporins into "generations" is commonly practised, although the exact categorisation of cephalosporins is often imprecise. For example, the fourth generation of cephalosporins is not yet recognized in Japan. In Japan, cefaclor is classed as a first generation cephalosporin; and cefbuperazone, cefminox and cefotetan are classed as second generation cephalosporins. Cefbuperazone, cefminox, and cefotetan are classed as second generation cephems. Cefmetazole and cefoxitin are classed as third generation cephems. Flomoxef, latamoxef are in a new class called oxacephems.
Most first generation cephalosporins were originally spelt "ceph-" in English-speaking countries. This continues to be the preferred spelling in North America and Australia, while European countries have adopted International Nonproprietary Names, which are usually spelt "cef-". Newer first-generation cephalosporins and all cephalosporins of later generations are spelt "cef-".
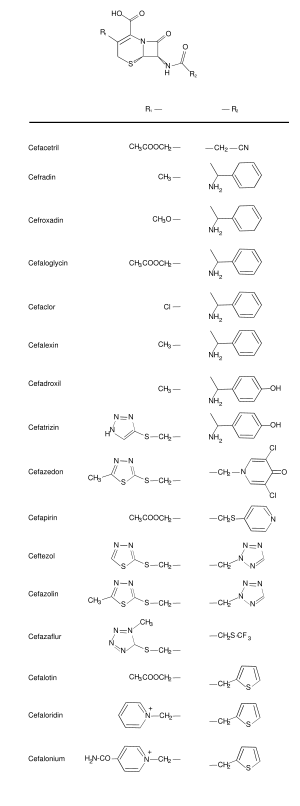
First generation
First generation cephalosporins are moderate spectrum agents, with a spectrum of activity that includes penicillinase-producing, methicillin-susceptible staphylococci and streptococci, though they are not the drugs of choice for such infections. They also have activity against some Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis, but have no activity against Bacteroides fragilis, enterococci, methicilllin-resistant staphylococci, Pseudomonas, Acinetobacter, Enterobacter, indole-positive Proteus or Serratia.
- Cefacetrile (cephacetrile)
Cefadroxil (cefadroxyl; Duricef®)
Cefalexin (cephalexin; Keflex®)
Cephaloglycin
Cefalonium (cephalonium)
Cefaloridine (cephaloradine)
Cefalotin (cephalothin; Keflin®)
Cefapirin (cephapirin; Cefadryl®)
Cefatrizine
Cefazaflur
Cefazedone
Cefazolin (cephazolin; Ancef®, Kefzol®)
Cefradine (cephradine; Velosef®)
Cefroxadine
Ceftezole
Second generation
The second generation cephalosporins have a greater Gram-negative spectrum while retaining some activity against Gram-positive cocci. They are also more resistant to beta-lactamase.
- Cefaclor (Ceclor®)
Cefonicid (Monocid®)
Cefprozil (cefproxil; Cefzil®)
Cefuroxime (Zinnat®, Zinacef®, Ceftin®)
Cefuzonam
Second generation cephalosporins with antianaerobe activity
- Cefamandole
Ceforanide
Cefotiam
The following cephems are also sometimes grouped with second-generation cephalosporins:
- Carbacephems: loracarbef (Lorabid®)
Cephamycins: cefbuperazone, cefmetazole (Zefazone®), cefminox, cefotetan (Cefotan®), cefoxitin (Mefoxin®)
Third generation
Third generation cephalosporins have a broad spectrum of activity and further increased activity against Gram-negative organisms. Some members of this group (particularly those available in an oral formulation, and those with anti-pseudomonal activity) have decreased activity against Gram-positive organisms. They may be particularly useful in treating hospital-acquired infections, although increasing levels of extended-spectrum beta-lactamases are reducing the clinical utility of this class of antibiotics.
- Cefcapene
Cefdaloxime
Cefdinir (Omnicef®)
Cefditoren
Cefetamet
Cefixime (Suprax®)
Cefmenoxime
Cefodizime
Cefoperazone (Cefobid®)
Cefotaxime (Claforan®)
Cefpimizole
Cefpodoxime (Vantin®)
Cefteram
Ceftibuten (Cedax®)
Ceftiofur
Ceftiolene
Ceftizoxime (Cefizax®)
Ceftriaxone (Rocephin®)
Third generation cephalosporins with antipseudomonal activity
- Ceftazidime (Fortum®, Fortaz®)
Cefpiramide
Cefsulodin
The following cephems are also sometimes grouped with third-generation cephalosporins:
- Oxacephems: latamoxef (moxalactam)
Fourth generation
Fourth generation cephalosporins are extended-spectrum agents with similar activity against Gram-positive organisms as first-generation cephalosporins. They also have a greater resistance to beta-lactamases than the third generation cephalosporins. Many can cross blood brain barrier and are effective in meningitis.
- Cefclidine
Cefepime (Maxipime®)
Cefluprenam
Cefoselis
Cefozopran
Cefpirome
Cefquinome
The following cephems are also sometimes grouped with third-generation cephalosporins:
- Oxacephems: flomoxef
Yet to be classified
These cephems have progressed far enough to be named, but have not been assigned to a particular generation. Ceftobiprole (and the oral medocaril version) are on the FDA fast track. Ceftobiprole has powerful antipseudomonal characteristics and appears to be less susceptible to development of resistance.
- Cefaclomezine
Cefaloram
Cefaparole
Cefcanel
Cefedrolor
Cefempidone
Cefetrizole
Cefivitril
Cefmatilen
Cefmepidium
Cefovecin
Cefoxazole
Cefrotil
Cefsumide
Ceftioxide
Ceftobiprole (previously BAL 9141 and RO 63-9141)
Ceftobiprole (previously BAL 5788)
Cefuracetime
References
- ^ Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006.
See also
External links
- MedlinePlus Drug Information: Cephalosporins (systemic) – information from USP DI Advice for the Patient
- Cephalosporins College of Health and Life Sciences, Fort Hays State University.
- Cephalosporins "Family Practice Notebook" page on Cephalosporins.
- Chemical Land 21 Cephalosporin class antibiotics
- Structure Activity Relationships "Antibacterial Agents; Structure Activity Relationships," André Bryskier MD; beginning at pp83




 216.73.216.81
216.73.216.81 User Stats:
User Stats:
 Today: 0
Today: 0 Yesterday: 0
Yesterday: 0 This Month: 0
This Month: 0 This Year: 0
This Year: 0 Total Users: 117
Total Users: 117 New Members:
New Members:
 216.73.xxx.xx
216.73.xxx.xx
 Server Time:
Server Time: